Choline Triggers Exacerbations of Chronic Obstructive Pulmonary Disease in Patients Infected with Pseudomonas aeruginosa
- PMID: 29386986
- PMCID: PMC5788026
- DOI: 10.2174/1573398X12999160506104327
Choline Triggers Exacerbations of Chronic Obstructive Pulmonary Disease in Patients Infected with Pseudomonas aeruginosa
Abstract
Background: Although exacerbations of chronic obstructive pulmonary disease produced by Pseudomonas aeruginosa infections are a major cause of death, the molecular mechanism that produces them is not well known. Here we focused on the energetic basis of dyspnoea, hypercapnia and acidosis symptoms.
Methods and findings: We used an in vivo exacerbation model exposing mice to cigarette smoke and LPS, to mimic emphysema and infections, and choline challenges to trigger exacerbations, that showed 31% increased in the airway resistance for naïve mice and 250% for smoke/LPS treatment. Tissue resistance was increased 32%, in naïve mice, and 169% for smoke/LPS treatment. A decreased tissue elastance, was confirmed by decreased collagen content and increased alveoli chord length. Consequently, the O2 demanded was 260% greater for smoke/LPS treated mice, to provide the energy required to pump the same volume of air then for naïve mice. The extra CO2 produced per ml of air pumped caused hypercapnia and acidosis by 4% decrease in pH.In addition, the bacteria grown with choline had a decrease of 67% in phosphate, 23% ATP and 85% phospholipids with an increase of 57% in polyphosphates, 50% carbohydrates, 100% LPS, consuming 45% less energy relative to the bacteria grown with succinate.
Conclusion: choline, released by P. aeruginosa, triggers exacerbation symptoms by increasing lung resistance, O2 consumption and producing more pCO2 in blood with dyspnea, hypercapnia and acidosis. The energetic shift of decreased O2 bacterial demand and increased lung demand benefits the infection, thus restoring the energetic balance on the host will favor P. aeruginosa eradication.
Keywords: Bacterial infections; COPD exacerbations; LPS; animal model; emphysema; respiratory infections.
Figures

 P= 0.01 relative to smoke exposed, §P=0.01 relative to naïve mice, †P=0.05 relative to smoke exposed, ∫P=0.05 relative to naïve mice, ‡P=0.01 relative to smoke exposed. (D) Plots show the pressure offered by the lung of mice smoke naïve (black circles, n=7), LPS (black squares, n=4), smokes exposed (open circles, n=4) and smoke plus LPS exposed (open squares, n=5) due to airway resistance (top plot) tissue resistance (middle plot) and dynamic elasticity (bottom plot). Data is expressed as mean+s.e.m, *P≤0.0001 determined by t-student test.
P= 0.01 relative to smoke exposed, §P=0.01 relative to naïve mice, †P=0.05 relative to smoke exposed, ∫P=0.05 relative to naïve mice, ‡P=0.01 relative to smoke exposed. (D) Plots show the pressure offered by the lung of mice smoke naïve (black circles, n=7), LPS (black squares, n=4), smokes exposed (open circles, n=4) and smoke plus LPS exposed (open squares, n=5) due to airway resistance (top plot) tissue resistance (middle plot) and dynamic elasticity (bottom plot). Data is expressed as mean+s.e.m, *P≤0.0001 determined by t-student test.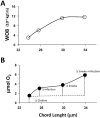
References
-
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. - PubMed
-
- Garcia-Vidal C, Almagro P, Romaní V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbations: a prospective study. Eur Respir J. 2009;34:1072–8. - PubMed
-
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180–86. - PubMed
-
- Laier-Groeneveld G, Gietl C, Bauer JU. Normocapnia following noninvasive ventilation in acute exacerbations and chronic state of obstructive pulmonary disese. J Physiol And Pharm G. 2007;58(Suppl 5):339–44. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
