Lauric Acid Is an Inhibitor of Clostridium difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model
- PMID: 29387044
- PMCID: PMC5776096
- DOI: 10.3389/fmicb.2017.02635
Lauric Acid Is an Inhibitor of Clostridium difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model
Abstract
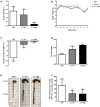
Clostridium difficile is a Gram-positive, spore-forming anaerobic human gastrointestinal pathogen. C. difficile infection (CDI) is a major health concern worldwide, with symptoms ranging from diarrhea to pseudomembranous colitis, toxic megacolon, sepsis, and death. CDI onset and progression are mostly caused by intestinal dysbiosis and exposure to C. difficile spores. Current treatment strategies include antibiotics; however, antibiotic use is often associated with high recurrence rates and an increased risk of antibiotic resistance. Medium-chain fatty acids (MCFAs) have been revealed to inhibit the growth of multiple human bacterial pathogens. Components of coconut oil, which include lauric acid, have been revealed to inhibit C. difficile growth in vitro. In this study, we demonstrated that lauric acid exhibits potent antimicrobial activities against multiple toxigenic C. difficile isolates in vitro. The inhibitory effect of lauric acid is partly due to reactive oxygen species (ROS) generation and cell membrane damage. The administration of lauric acid considerably reduced biofilm formation and preformed biofilms in a dose-dependent manner. Importantly, in a mouse infection model, lauric acid pretreatment reduced CDI symptoms and proinflammatory cytokine production. Our combined results suggest that the naturally occurring MCFA lauric acid is a novel C. difficile inhibitor and is useful in the development of an alternative or adjunctive treatment for CDI.
Keywords: Clostridium difficile; alternative therapy; lauric acid; medium-chain fatty acid; natural product.
Figures

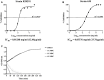
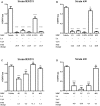
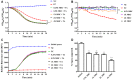
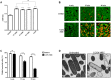
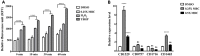

Similar articles
-
Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile.J Med Food. 2013 Dec;16(12):1079-85. doi: 10.1089/jmf.2012.0303. J Med Food. 2013. PMID: 24328700
-
Clostridium difficile, the Difficult "Kloster" Fuelled by Antibiotics.Curr Microbiol. 2019 Jun;76(6):774-782. doi: 10.1007/s00284-018-1543-8. Epub 2018 Aug 6. Curr Microbiol. 2019. PMID: 30084095 Review.
-
Clostridium difficile Biofilm.Adv Exp Med Biol. 2018;1050:97-115. doi: 10.1007/978-3-319-72799-8_7. Adv Exp Med Biol. 2018. PMID: 29383666 Review.
-
[Clostridium difficile infection: epidemiology, risk factors, pathogenesis, clinical features, diagnosis and therapy].Mikrobiyol Bul. 2013 Jul;47(3):556-66. doi: 10.5578/mb.5208. Mikrobiyol Bul. 2013. PMID: 23971935 Review. Turkish.
-
High sporulation and overexpression of virulence factors in biofilms and reduced susceptibility to vancomycin and linezolid in recurrent Clostridium [Clostridioides] difficile infection isolates.PLoS One. 2019 Jul 31;14(7):e0220671. doi: 10.1371/journal.pone.0220671. eCollection 2019. PLoS One. 2019. PMID: 31365590 Free PMC article.
Cited by
-
Improving Anti-listeria Activity of Thymol Emulsions by Adding Lauric Acid.Front Nutr. 2022 Apr 8;9:859293. doi: 10.3389/fnut.2022.859293. eCollection 2022. Front Nutr. 2022. PMID: 35464037 Free PMC article.
-
Effective inhibition of Clostridioides difficile by the novel peptide CM-A.PLoS One. 2021 Sep 13;16(9):e0257431. doi: 10.1371/journal.pone.0257431. eCollection 2021. PLoS One. 2021. PMID: 34516580 Free PMC article.
-
A Tea Polyphenol-Infused Sprayable Thermosensitive Liposomal Hydrogel for Enhanced Anti-Inflammatory and Antibacterial Psoriasis Treatment.J Funct Biomater. 2025 Apr 1;16(4):124. doi: 10.3390/jfb16040124. J Funct Biomater. 2025. PMID: 40278232 Free PMC article.
-
Insect Derived Lauric Acid as Promising Alternative Strategy to Antibiotics in the Antimicrobial Resistance Scenario.Front Microbiol. 2021 Feb 26;12:620798. doi: 10.3389/fmicb.2021.620798. eCollection 2021. Front Microbiol. 2021. PMID: 33717009 Free PMC article. Review.
-
Clostridioides difficile: innovations in target discovery and potential for therapeutic success.Expert Opin Ther Targets. 2021 Nov;25(11):949-963. doi: 10.1080/14728222.2021.2008907. Epub 2021 Dec 2. Expert Opin Ther Targets. 2021. PMID: 34793686 Free PMC article.
References
-
- Bartolotta S., Garcia C. C., Candurra N. A., Damonte E. B. (2001). Effect of fatty acids on arenavirus replication: inhibition of virus production by lauric acid. Arch. Virol. 146 777–790. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

