Salmonella Typhi Bactericidal Antibodies Reduce Disease Severity but Do Not Protect against Typhoid Fever in a Controlled Human Infection Model
- PMID: 29387052
- PMCID: PMC5776093
- DOI: 10.3389/fimmu.2017.01916
Salmonella Typhi Bactericidal Antibodies Reduce Disease Severity but Do Not Protect against Typhoid Fever in a Controlled Human Infection Model
Abstract
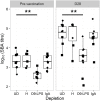
Effective vaccines against Salmonella Typhi, a major cause of febrile illness in tropical regions, can have a significant effect as a disease control measure. Earlier work has shown that immunization with either of two Salmonella Typhi vaccines, licensed Ty21a or candidate M01ZH09, did not provide full immunity in a controlled human infection model. Here, we describe the human humoral immune responses to these oral vaccines and their functional role in protection after challenge with S. Typhi. Serum, obtained from healthy volunteers before and after vaccination with Ty21a or M01ZH09 or placebo and before and after oral challenge with wild-type S. Typhi, was assessed for bactericidal activity. Single-dose vaccination with M01ZH09 induced an increase in serum bactericidal antibodies (p = 0.001) while three doses of Ty21a did not. No association between bactericidal activity and protection against typhoid after challenge was seen in either vaccine arm. Bactericidal activity after vaccination correlated significantly with delayed disease onset (p = 0.013), lower bacterial burden (p = 0.006), and decreased disease severity scores (p = 0.021). Depletion of antibodies directed against lipopolysaccharide significantly reduced bactericidal activity (p = 0.009). We conclude that antibodies induced after ingestion of oral live-attenuated typhoid vaccines or after challenge with wild-type S. Typhi exhibit bactericidal activity. This bactericidal activity is mediated by anti-O:LPS antibodies and significantly reduces clinical symptoms but does not provide sterile immunity. This directs future vaccine studies toward other antigens or mechanisms of protection against typhoid.
Keywords: Salmonella enterica Typhi; bactericidal activity; human challenge model; immune responses; typhoid infection.
Figures



References
-
- WHO. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec (2008) 83:49–59. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

