Brucella Downregulates Tumor Necrosis Factor-α to Promote Intracellular Survival via Omp25 Regulation of Different MicroRNAs in Porcine and Murine Macrophages
- PMID: 29387067
- PMCID: PMC5776175
- DOI: 10.3389/fimmu.2017.02013
Brucella Downregulates Tumor Necrosis Factor-α to Promote Intracellular Survival via Omp25 Regulation of Different MicroRNAs in Porcine and Murine Macrophages
Abstract
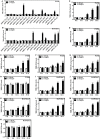
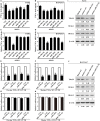
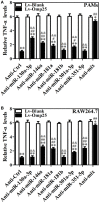
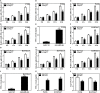
Brucella spp. impedes the production of pro-inflammatory cytokines by its outer membrane protein Omp25 in order to promote survival and immune evasion. However, how Omp25 regulates tumor necrosis factor (TNF-α) expression in different mammalian macrophages remains unclear. In this study, we investigated the potential mechanisms by which Omp25 regulates TNF-α expression and found that Omp25-deficient mutant of B. suis exhibited an enhanced TNF-α expression compared with wild-type (WT) B. suis, whereas ectopic expression of Omp25 suppressed LPS-induced TNF-α production at both protein and mRNA levels in porcine alveolar macrophages (PAMs) and murine macrophage RAW264.7 cells. We observed that Omp25 protein as well as WT B. suis upregulated miR-146a, -181a, -181b, and -301a-3p and downregulated TRAF6 and IRAK1 in both PAMs and RAW264.7 cells, but separately upregulates miR-130a-3p in PAMs and miR-351-5p in RAW264.7. The upregulation of miR-146a or miR-351-5p attenuated TNF-α transcription by targeting TRAF6 and IRAK1 at the 3' untranslated region (UTR), resulting in inhibition of NF-kB pathway, while upregulation of miR-130a-3p, -181a, or -301a-3p correlated temporally with decreased TNF-α by targeting its 3'UTR in PAMs or RAW264.7 cells. In contrast, inhibition of miR-130a-3p, -146a, -181a, and -301a-3p attenuated the inhibitory effects of Omp25 on LPS-induced TNF-α in PAMs, while inhibition of miR-146a, -181a, -301a-3p, and -351-5p attenuated the inhibitory effects of Omp25 in RAW264.7, resulting in an increased TNF-α production and decreased intracellular bacteria in both cells. Taken together, our results demonstrate that Brucella downregulates TNF-α to promote intracellular survival via Omp25 regulation of different microRNAs in porcine and murine macrophages.
Keywords: Brucella suis; Omp25; macrophage; miRNA; tumor necrosis factor-α.
Figures





Similar articles
-
Brucella Omp25 Upregulates miR-155, miR-21-5p, and miR-23b to Inhibit Interleukin-12 Production via Modulation of Programmed Death-1 Signaling in Human Monocyte/Macrophages.Front Immunol. 2017 Jun 26;8:708. doi: 10.3389/fimmu.2017.00708. eCollection 2017. Front Immunol. 2017. PMID: 28694807 Free PMC article.
-
Major outer membrane protein Omp25 of Brucella suis is involved in inhibition of tumor necrosis factor alpha production during infection of human macrophages.Infect Immun. 2001 Aug;69(8):4823-30. doi: 10.1128/IAI.69.8.4823-4830.2001. Infect Immun. 2001. PMID: 11447156 Free PMC article.
-
Integrative Bioinformatics Indentification of the Autophagic Pathway-Associated miRNA-mRNA Networks in RAW264.7 Macrophage Cells Infected with ∆Omp25 Brucella melitensis.Inflammation. 2020 Apr;43(2):532-539. doi: 10.1007/s10753-019-01135-6. Inflammation. 2020. PMID: 31807961
-
The innate immune response against Brucella in humans.Vet Microbiol. 2002 Dec 20;90(1-4):383-94. doi: 10.1016/s0378-1135(02)00223-7. Vet Microbiol. 2002. PMID: 12414158 Review.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
Cited by
-
Interleukin-10 Promotes Porcine Circovirus Type 2 Persistent Infection in Mice and Aggravates the Tissue Lesions by Suppression of T Cell Infiltration.Front Microbiol. 2019 Sep 10;10:2050. doi: 10.3389/fmicb.2019.02050. eCollection 2019. Front Microbiol. 2019. PMID: 31551984 Free PMC article.
-
Host F-Box Protein 22 Enhances the Uptake of Brucella by Macrophages and Drives a Sustained Release of Proinflammatory Cytokines through Degradation of the Anti-Inflammatory Effector Proteins of Brucella.Infect Immun. 2022 May 19;90(5):e0006022. doi: 10.1128/iai.00060-22. Epub 2022 Apr 14. Infect Immun. 2022. PMID: 35420446 Free PMC article.
-
Wilful pathogens provoke a gut feeling in Parkinson's disease.J Neural Transm (Vienna). 2022 Jun;129(5-6):557-562. doi: 10.1007/s00702-021-02448-3. Epub 2021 Dec 19. J Neural Transm (Vienna). 2022. PMID: 34923593 Free PMC article. Review.
-
Immunobiology of cancer stem cells and their immunoevasion mechanisms.Mol Biol Rep. 2023 Nov;50(11):9559-9573. doi: 10.1007/s11033-023-08768-9. Epub 2023 Sep 30. Mol Biol Rep. 2023. PMID: 37776412 Review.
-
RNA-Seq Analysis Reveals the Role of Omp16 in Brucella-Infected RAW264.7 Cells.Front Vet Sci. 2021 Mar 4;8:646839. doi: 10.3389/fvets.2021.646839. eCollection 2021. Front Vet Sci. 2021. PMID: 33748220 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

