A Novel L-ascorbate Peroxidase 6 Gene, ScAPX6, Plays an Important Role in the Regulation of Response to Biotic and Abiotic Stresses in Sugarcane
- PMID: 29387074
- PMCID: PMC5776131
- DOI: 10.3389/fpls.2017.02262
A Novel L-ascorbate Peroxidase 6 Gene, ScAPX6, Plays an Important Role in the Regulation of Response to Biotic and Abiotic Stresses in Sugarcane
Abstract
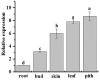
The L-ascorbate peroxidase 6 gene (APX6) is one of the most important genes for scavenging H2O2 and plays a vital role in plant resistance to environmental stresses. In this study, a novel ScAPX6 gene (GenBank Accession No. KT907352) was obtained from a sugarcane variety (ROC22). Bioinformatics analysis showed that ScAPX6 has a cDNA length of 1,086 bp and encoded 333 amino acid residues. Subcellular localization confirmed that ScAPX6 was located in the chloroplast. Enhanced growth of Escherichia coli BL21 cells that expressed ScAPX6 showed high tolerance under copper (Cu) stress. Real-time quantitative PCR analysis revealed that ScAPX6 was constitutively expressed wherein with the highest expression levels in sugarcane pith and leaf and the lowest in the root. ScAPX6 was down-regulated by salicylic acid (SA), hydrogen peroxide (H2O2), polyethylene glycol (PEG) and sodium chloride (NaCl) stimuli. Interestingly, it was significantly up-regulated under the stresses of abscisic acid (ABA) and methyl jasmonate (MeJA) wherein with the highest inducible expression levels at 6 h at 6.0- and 70.0-times higher, respectively than that of control. Overexpression of ScAPX6 in Nicotiana benthamiana leaves enhanced the resistance to the infection of tobacco pathogens Pseudomonas solanacearum and Fusarium solani var. coeruleum. These results implied that ScAPX6 might positively respond to ABA, MeJA, and Cu, but might negatively respond to the stresses of SA, H2O2, PEG, and NaCl. Keeping in view the current investigation, ScAPX6 could be associated with the hypersensitive response (HR) or immunity of sugarcane, which will provide a baseline for the function identification of sugarcane ScAPX6.
Keywords: L-ascorbate peroxidase 6 gene; biotic and abiotic stresses; subcellular localization; sugarcane; transient overexpression.
Figures







Similar articles
-
ScAOC1, an allene oxide cyclase gene, confers defense response to biotic and abiotic stresses in sugarcane.Plant Cell Rep. 2020 Dec;39(12):1785-1801. doi: 10.1007/s00299-020-02606-z. Epub 2020 Oct 1. Plant Cell Rep. 2020. PMID: 33001313
-
The Role of Sugarcane Catalase Gene ScCAT2 in the Defense Response to Pathogen Challenge and Adversity Stress.Int J Mol Sci. 2018 Sep 10;19(9):2686. doi: 10.3390/ijms19092686. Int J Mol Sci. 2018. PMID: 30201878 Free PMC article.
-
Sugarcane calcineurin B-like (CBL) genes play important but versatile roles in regulation of responses to biotic and abiotic stresses.Sci Rep. 2020 Jan 13;10(1):167. doi: 10.1038/s41598-019-57058-7. Sci Rep. 2020. PMID: 31932662 Free PMC article.
-
A sugarcane pathogenesis-related protein, ScPR10, plays a positive role in defense responses under Sporisorium scitamineum, SrMV, SA, and MeJA stresses.Plant Cell Rep. 2017 Sep;36(9):1427-1440. doi: 10.1007/s00299-017-2166-4. Epub 2017 Jun 20. Plant Cell Rep. 2017. PMID: 28634719
-
Expression Characteristics and Functional Analysis of the ScWRKY3 Gene from Sugarcane.Int J Mol Sci. 2018 Dec 14;19(12):4059. doi: 10.3390/ijms19124059. Int J Mol Sci. 2018. PMID: 30558233 Free PMC article.
Cited by
-
Transcriptional Regulation of SugarCane Response to Sporisorium scitamineum: Insights from Time-Course Gene Coexpression and Ca2+ Signaling.J Agric Food Chem. 2024 May 8;72(18):10506-10520. doi: 10.1021/acs.jafc.4c02123. Epub 2024 Apr 23. J Agric Food Chem. 2024. PMID: 38651833 Free PMC article.
-
Identification and Characterization of the APX Gene Family and Its Expression Pattern under Phytohormone Treatment and Abiotic Stress in Populus trichocarpa.Genes (Basel). 2021 Feb 25;12(3):334. doi: 10.3390/genes12030334. Genes (Basel). 2021. PMID: 33668872 Free PMC article.
-
Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress.Antioxidants (Basel). 2019 Sep 9;8(9):384. doi: 10.3390/antiox8090384. Antioxidants (Basel). 2019. PMID: 31505852 Free PMC article. Review.
-
Multiomics analysis reveals the molecular mechanisms underlying virulence in Rhizoctonia and jasmonic acid-mediated resistance in Tartary buckwheat (Fagopyrum tataricum).Plant Cell. 2023 Aug 2;35(8):2773-2798. doi: 10.1093/plcell/koad118. Plant Cell. 2023. PMID: 37119263 Free PMC article.
-
Genome-Wide Characterization of Ascorbate Peroxidase Gene Family in Peanut (Arachis hypogea L.) Revealed Their Crucial Role in Growth and Multiple Stress Tolerance.Front Plant Sci. 2022 Sep 9;13:962182. doi: 10.3389/fpls.2022.962182. eCollection 2022. Front Plant Sci. 2022. PMID: 36186077 Free PMC article.
References
-
- Agarwal S., Sairam R. K., Srivastava G. C., Tyagi A., Meena R. C. (2005). Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 169, 559–570. 10.1016/j.plantsci.2005.05.004 - DOI
-
- Ahmad R., Kim Y. H., Kim M. D., Kwon S. Y., Cho K., Lee H. S., et al. . (2010). Simultaneous expression of choline oxidase, superoxide dismutase and ascorbate peroxidase in potato plant chloroplasts provides synergistically enhanced protection against various abiotic stresses. Physiol. Plant. 138:520. 10.1111/j.1399-3054.2010.01348.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

