Effects of different activation protocols on cleavage rate and blastocyst production of caprine oocytes
- PMID: 29387095
- PMCID: PMC5767629
Effects of different activation protocols on cleavage rate and blastocyst production of caprine oocytes
Abstract
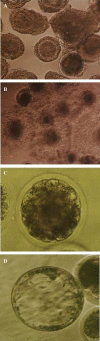
The present study was undertaken to assess the effect of different chemical activators along with 6-DMAP on in vitro matured caprine oocytes. From 4332 ovaries, 14235 cumulus oocyte complexes (COCs) were collected which were matured in TCM-199 medium containing follicle stimulating hormone (FSH) (5 µg/ml), Leutinizing hormone (LH) (10 µg/ml), oestradiol-17β (1 µg/ml) supplemented with 10% fetal bovine serum, 10% follicular fluid and 3 mg/ml bovine serum albumin (BSA) at 38.5°C and 5% CO2 in an incubator under humidified air for 27 h. In group 1 (control), 3117 in vitro matured oocytes were co incubated with sperms for 18 h in ferttalp medium. In group 2, 3563 in vitro matured oocytes were activated with 7% ethanol for 5-7 min followed by treatment with 2.0 mM DMAP for 4 h in mCR2aa medium. In group 3, 3109 in vitro matured oocytes were activated with 5 μM ionomycin for 5-7 min followed by treatment with 2.0 mM DMAP for 4 h in mCR2aa medium. In group 4, 3455 in vitro matured oocytes were activated with 5 μM calcium ionophore for 5-7 min followed by treatment with 2.0 mM DMAP for 4 h in mCR2aa medium. Oocytes were cultured in 50 µL drops of research vitro cleave (RVCL) medium for embryo development. The cleavage rate, morula and blastocyst production in group 1, 2, 3 and 4 were 26.07 ± 2.37%, 14.91 ± 2.91 & 1.45 ± 0.71%, 49.57 ± 3.79%, 20.07 ± 2.38% & 5.29 ± 1.42%, 50.18 ± 3.59%, 15.26 ± 2.87% & 1.85 ± 0.72% and 80.26 ± 2.30%, 35.33 ± 2.67 & 7.10 ± 0.89%, respectively. These results indicated that the activation of in vitro matured oocytes by 5 μM calcium ionophore for 5-7 min followed by treatment with 2.0 mM DMAP for 4 h is most favorable for parthenogenetic caprine embryos production.
Keywords: Blastocyst; Calcium ionophore; Caprine; Cleavage; Parthenogenesis.
Conflict of interest statement
None of the authors have any conflict of interest to declare.
Figures
Similar articles
-
Effect of different activators on development of activated in vitro matured caprine oocytes.Iran J Vet Res. 2015 Winter;16(1):42-6. Iran J Vet Res. 2015. PMID: 27175149 Free PMC article.
-
A comparative study of parthenogenetic activation and in vitro fertilization of in vitro matured caprine oocytes.Iran J Vet Res. 2015 Winter;16(1):20-4. Iran J Vet Res. 2015. PMID: 27175145 Free PMC article.
-
Effect of Ca Ionophore On Blastocyst Production Following Intracytoplasmic Sperm Injection in Caprine Oocytes.Reprod Domest Anim. 2016 Aug;51(4):611-7. doi: 10.1111/rda.12701. Epub 2016 May 11. Reprod Domest Anim. 2016. PMID: 27170442
-
Morphology of dromedary camel oocytes and their ability to spontaneous and chemical parthenogenetic activation.Reprod Domest Anim. 2007 Feb;42(1):88-93. doi: 10.1111/j.1439-0531.2006.00737.x. Reprod Domest Anim. 2007. PMID: 17214780
-
Calcium ionophore enhanced developmental competence and apoptotic dynamics of goat parthenogenetic embryos produced in vitro.In Vitro Cell Dev Biol Anim. 2019 Mar;55(3):159-168. doi: 10.1007/s11626-019-00322-x. Epub 2019 Feb 8. In Vitro Cell Dev Biol Anim. 2019. PMID: 30737632
Cited by
-
Exploration of the Cytoplasmic Function of Abnormally Fertilized Embryos via Novel Pronuclear-Stage Cytoplasmic Transfer.Int J Mol Sci. 2021 Aug 16;22(16):8765. doi: 10.3390/ijms22168765. Int J Mol Sci. 2021. PMID: 34445470 Free PMC article.
-
Oocyte Penetration Speed Optimization Based on Intracellular Strain.Micromachines (Basel). 2022 Feb 17;13(2):309. doi: 10.3390/mi13020309. Micromachines (Basel). 2022. PMID: 35208433 Free PMC article.
-
Temperature response of enriched pre-pubertal caprine male germline stem cells in vitro.Cell Stress Chaperones. 2021 Nov;26(6):989-1000. doi: 10.1007/s12192-021-01236-y. Epub 2021 Sep 22. Cell Stress Chaperones. 2021. PMID: 34553319 Free PMC article.
References
-
- Abdoon AS, Ghanem N, Kandila OM, Gad A, Schellander K, Tesfaye D. cDNA microarray analysis of gene expression in parthenotes and in vitro produced buffalo embryos. Theriogenology. 2012;77:1240–1251. - PubMed
-
- Abdullah RB, Wan Khadijah WE, Kwong PJ. Comparison of intra- and interspecies nuclear transfer techniques in the production of cloned caprine embryos. Small Rumin. Res. 2011;98:196–200.
-
- De AK, Garg S, Singhala DK, Malika H, Mukherjee A, Jena MK, Kumar S, Kaushik JK, Mohanty AK, Das BC, Bag S, Bhanja SK, Malakar D. Derivation of goat embryonic stem cell-like cell lines from in vitro produced parthenogenetic blastocysts. Small Rumin. Res. 2013;113:145–153.
-
- De LFR, King WA. Developmental consequences of karyokinesis without cytokinesis during the first mitotic cell cycle of bovine parthenotes. Biol. Reprod. 1998;58:952–962. - PubMed
-
- De AK, Malakar D, Jena MK, Dutta R, Garg S, Akshey YS. Zona-free and with-zonaparthenogenetic embryo production in goat (Capra hircus) - effect of activation methods, culture systems and culture media. Livest. Sci. 2012;143:35–42.
LinkOut - more resources
Full Text Sources