IL-1β increases urinary corin in patients with primary proteinuric kidney diseases and in 293 cells
- PMID: 29387201
- PMCID: PMC5769225
- DOI: 10.3892/etm.2017.5398
IL-1β increases urinary corin in patients with primary proteinuric kidney diseases and in 293 cells
Abstract
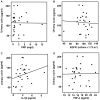
Corin is a serine protease that is important for the regulation of blood pressure and water balance. Corin was initially discovered in the heart, however, it has also been detected in kidney cells, though its function in the kidneys is unclear. To further investigate the function of corin in the kidney, the present study analyzed the levels of corin in urine and blood samples collected from normal individuals and patients with primary proteinuric diseases. The associations between the levels of corin, and the cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were then assessed. The results demonstrated that corin was detectable in the urine and plasma following an enzyme-linked immunosorbent assay; the level of corin in the urine was associated with the level of urinary β2-microglobulin (P=0.01), which was indicative of renal tubular injury. When compared with normal individuals, the levels of urinary corin in proteinuric patients were markedly increased (P=0.02), and were also associated with IL-1β (P=0.03). This correlation between corin and IL-1β was confirmed in vitro using 293 cells. As the IL-1β concentrations increased (0, 0.1, 1, 10 ng/ml), an elevation in the level of corin was observed in the culture medium (P<0.01); however, the amount of corin was not markedly altered in the cell lysate (P>0.05). In addition, when TNF-α reached 10 ng/ml, the level of corin in the medium increased significantly when compared with the control group (0 ng/ml; P=0.02), however, no significant difference in corin levels was detected in the cell lysate. The results suggest that the cytokines IL-1β and TNF-α may increase urinary corin in patients with primary proteinuric kidney diseases. Cytokines may accelerate corin shedding from the cell membrane of renal tubule epithelial cells. These findings indicate that corin may be associated with kidney inflammation and injury.
Keywords: chronic glomerulonephritis; corin protein; cytokines; interleukin-1β; tumor necrosis factor-α.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
