Prodrugs of NSAIDs: A Review
- PMID: 29387273
- PMCID: PMC5748882
- DOI: 10.2174/1874104501711010146
Prodrugs of NSAIDs: A Review
Abstract
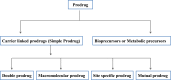
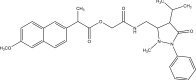
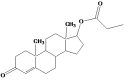
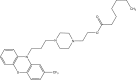
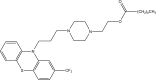
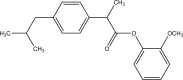
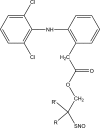
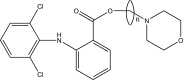
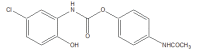
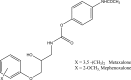
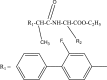
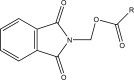
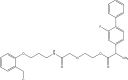
Intoroduction: Prodrug approach deals with chemical biotransformation or enzymatic conversion or involves inactive or less active bio-reversible derivatives of active drug molecules. They have to pass through enzymatic or chemical biotransformation before eliciting their pharmacological action.
Methods & materials: The two different pharmacophores combine to give synergistic activity or may help in targeting the active drug to its target. Prodrug super seeds the problems of prodrug designing, for example solubility enhancement, bioavailability enhancement, chemical stability improvement, presystemic metabolism, site specific delivery, toxicity masking, improving patient acceptance, or eradicating undesirable adverse effects.
Results: As an outcome the search for a prodrug or mutual prodrug with reduced toxicity has continued during recent years. This present review emphasizes the common help to revamp physiochemical, pharmaceutical and therapeutic effectiveness of drugs.
Conclusion: This gives the researcher a common platform where they can find prodrugs of commonly used NSAIDs to overcome the gastrointestinal toxicity (irritation, ulcergenocity and bleeding).
Keywords: Enzymatic attack; Gastrointestinal toxicity; NSAIDs; Prodrug; Synergistic; Ulcergenocity.
Figures

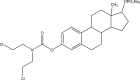

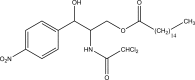


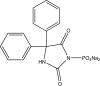

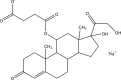






.gif)
.gif)

.gif)
.gif)

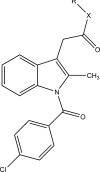
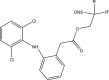

.gif)
.gif)

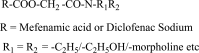

.gif)
.gif)
.gif)
.gif)
.gif)
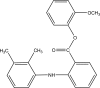
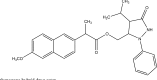



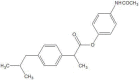
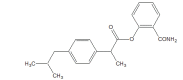

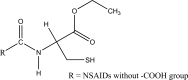




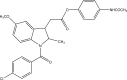
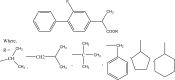



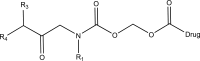

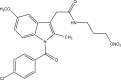
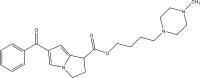


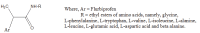
.gif)
.gif)
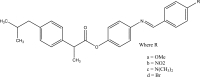


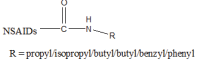
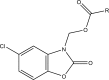
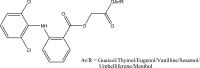
.gif)
.gif)
.gif)
.gif)
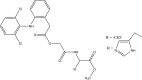




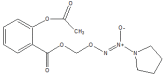


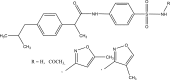
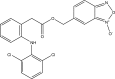



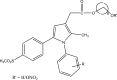

.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
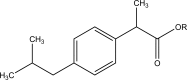
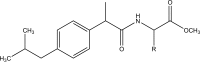
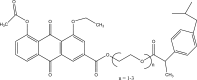
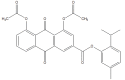

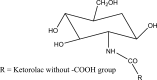
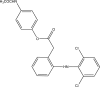
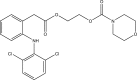
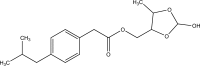


References
-
- Notari RE. Biopharmaceutics and Clinical Pharmacokinetics Marcel Dekker New York. 1987. pp. 316–318.
-
- Stella VJ, Borchardt RT, Hageman MJ, Oliyai R, Maag H, Tilley JW. Prodrugs. Vol. 37. New York. Springer Science: Challenges and Rewards Part 1 and 2; 2007.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
