Improved in Vitro Folding of the Y2 G Protein-Coupled Receptor into Bicelles
- PMID: 29387686
- PMCID: PMC5776092
- DOI: 10.3389/fmolb.2017.00100
Improved in Vitro Folding of the Y2 G Protein-Coupled Receptor into Bicelles
Abstract
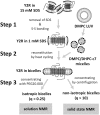
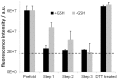
Prerequisite for structural studies on G protein-coupled receptors is the preparation of highly concentrated, stable, and biologically active receptor samples in milligram amounts of protein. Here, we present an improved protocol for Escherichia coli expression, functional refolding, and reconstitution into bicelles of the human neuropeptide Y receptor type 2 (Y2R) for solution and solid-state NMR experiments. The isotopically labeled receptor is expressed in inclusion bodies and purified using SDS. We studied the details of an improved preparation protocol including the in vitro folding of the receptor, e.g., the native disulfide bridge formation, the exchange of the denaturating detergent SDS, and the functional reconstitution into bicelle environments of varying size. Full pharmacological functionality of the Y2R preparation was shown by a ligand affinity of 4 nM and G-protein activation. Further, simple NMR experiments are used to test sample quality in high micromolar concentration.
Keywords: GPCR; NMR; NPY; bicelles; folding.
Figures





Similar articles
-
A reconstitution protocol for the in vitro folded human G protein-coupled Y2 receptor into lipid environment.Biophys Chem. 2010 Aug;150(1-3):29-36. doi: 10.1016/j.bpc.2010.02.019. Epub 2010 Apr 8. Biophys Chem. 2010. PMID: 20421142
-
Prokaryotic expression, in vitro folding, and molecular pharmacological characterization of the neuropeptide Y receptor type 2.Biotechnol Prog. 2009 Nov-Dec;25(6):1732-9. doi: 10.1002/btpr.266. Biotechnol Prog. 2009. PMID: 19725122
-
Unwinding of the C-Terminal Residues of Neuropeptide Y is critical for Y₂ Receptor Binding and Activation.Angew Chem Int Ed Engl. 2015 Jun 15;54(25):7446-9. doi: 10.1002/anie.201411688. Epub 2015 Apr 29. Angew Chem Int Ed Engl. 2015. PMID: 25924821 Free PMC article.
-
Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family.J Pept Sci. 2000 Mar;6(3):97-122. doi: 10.1002/(SICI)1099-1387(200003)6:3<97::AID-PSC236>3.0.CO;2-E. J Pept Sci. 2000. PMID: 10759209 Review.
-
Neuropeptide Y receptor subtypes.Life Sci. 1995 Feb 17;56(13):1055-64. doi: 10.1016/0024-3205(95)00041-4. Life Sci. 1995. PMID: 9001438 Review.
Cited by
-
Expression and Preparation of a G-Protein-Coupled Cannabinoid Receptor CB2 for NMR Structural Studies.Curr Protoc Protein Sci. 2019 Jun;96(1):e83. doi: 10.1002/cpps.83. Epub 2019 Jan 9. Curr Protoc Protein Sci. 2019. PMID: 30624864 Free PMC article.
-
Rhomboid-catalyzed intramembrane proteolysis requires hydrophobic matching with the surrounding lipid bilayer.Sci Adv. 2022 Sep 23;8(38):eabq8303. doi: 10.1126/sciadv.abq8303. Epub 2022 Sep 23. Sci Adv. 2022. PMID: 36149963 Free PMC article.
-
Cell-Free Expression and Photo-Crosslinking of the Human Neuropeptide Y2 Receptor.Front Pharmacol. 2019 Mar 1;10:176. doi: 10.3389/fphar.2019.00176. eCollection 2019. Front Pharmacol. 2019. PMID: 30881304 Free PMC article.
-
Integration of Cell-Free Expression and Solid-State NMR to Investigate the Dynamic Properties of Different Sites of the Growth Hormone Secretagogue Receptor.Front Pharmacol. 2020 Oct 29;11:562113. doi: 10.3389/fphar.2020.562113. eCollection 2020. Front Pharmacol. 2020. PMID: 33324203 Free PMC article.
-
Neuropeptide Y Peptide Family and Cancer: Antitumor Therapeutic Strategies.Int J Mol Sci. 2023 Jun 9;24(12):9962. doi: 10.3390/ijms24129962. Int J Mol Sci. 2023. PMID: 37373115 Free PMC article. Review.
References
-
- Baneres J. L., Martin A., Hullot P., Girard J. P., Rossi J. C., Parello J. (2003). Structure-based analysis of GPCR function: conformational adaptation of both agonist and receptor upon leukotriene B4 binding to recombinant BLT1. J. Mol. Biol. 329, 801–814. 10.1016/S0022-2836(03)00438-8 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

