Decellularized Swine Dental Pulp as a Bioscaffold for Pulp Regeneration
- PMID: 29387727
- PMCID: PMC5745671
- DOI: 10.1155/2017/9342714
Decellularized Swine Dental Pulp as a Bioscaffold for Pulp Regeneration
Abstract
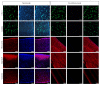
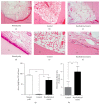
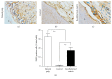
Endodontic regeneration shows promise in treating dental pulp diseases; however, no suitable scaffolds exist for pulp regeneration. Acellular natural extracellular matrix (ECM) is a favorable scaffold for tissue regeneration since the anatomical structure and ECM of the natural tissues or organs are well-preserved. Xenogeneic ECM is superior to autologous or allogeneic ECM in tissue engineering for its unlimited resources. This study investigated the characteristics of decellularized dental pulp ECM from swine and evaluated whether it could mediate pulp regeneration. Dental pulps were acquired from the mandible anterior teeth of swine 12 months of age and decellularized with 10% sodium dodecyl sulfate (SDS) combined with Triton X-100. Pulp regeneration was conducted by seeding human dental pulp stem cells into decellularized pulp and transplanted subcutaneously into nude mice for 8 weeks. The decellularized pulp demonstrated preserved natural shape and structure without any cellular components. Histological analysis showed excellent ECM preservation and pulp-like tissue, and newly formed mineralized tissues were regenerated after being transplanted in vivo. In conclusion, decellularized swine dental pulp maintains ECM components favoring stem cell proliferation and differentiation, thus representing a suitable scaffold for improving clinical outcomes and functions of teeth with dental pulp diseases.
Figures

Similar articles
-
Decellularized Swine Dental Pulp Tissue for Regenerative Root Canal Therapy.J Dent Res. 2018 Dec;97(13):1460-1467. doi: 10.1177/0022034518785124. Epub 2018 Aug 1. J Dent Res. 2018. PMID: 30067420
-
Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration.Biomaterials. 2015 Jun;52:56-70. doi: 10.1016/j.biomaterials.2015.02.011. Epub 2015 Feb 21. Biomaterials. 2015. PMID: 25818413
-
A biocompatible decellularized pulp scaffold for regenerative endodontics.Int Endod J. 2018 Jun;51(6):663-673. doi: 10.1111/iej.12882. Epub 2017 Dec 16. Int Endod J. 2018. PMID: 29197101
-
Organ reconstruction: Dream or reality for the future.Biomed Mater Eng. 2017;28(s1):S121-S127. doi: 10.3233/BME-171633. Biomed Mater Eng. 2017. PMID: 28372287 Review.
-
Influence of extracellular matrix scaffolds on histological outcomes of regenerative endodontics in experimental animal models: a systematic review.BMC Oral Health. 2024 Apr 30;24(1):511. doi: 10.1186/s12903-024-04266-x. BMC Oral Health. 2024. PMID: 38689279 Free PMC article.
Cited by
-
Future of Decellularized Dental Pulp Matrix in Regenerative Endodontics.Eur J Dent. 2022 Oct;16(4):737-741. doi: 10.1055/s-0041-1741012. Epub 2022 Jan 6. Eur J Dent. 2022. PMID: 34991166 Free PMC article.
-
Potential Research Tool of Stem Cells from Human Exfoliated Deciduous Teeth: Lentiviral Bmi-1 Immortalization with EGFP Marker.Stem Cells Int. 2019 Mar 10;2019:3526409. doi: 10.1155/2019/3526409. eCollection 2019. Stem Cells Int. 2019. PMID: 30984268 Free PMC article.
-
The Regenerative Potential of Decellularized Dental Pulp Extracellular Matrix: A Systematic Review.Materials (Basel). 2022 Sep 14;15(18):6386. doi: 10.3390/ma15186386. Materials (Basel). 2022. PMID: 36143698 Free PMC article. Review.
-
Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy.Biomed Res Int. 2019 Jan 29;2019:6104738. doi: 10.1155/2019/6104738. eCollection 2019. Biomed Res Int. 2019. PMID: 30834270 Free PMC article. Review.
-
A narrative overview of utilizing biomaterials to recapitulate the salient regenerative features of dental-derived mesenchymal stem cells.Int J Oral Sci. 2021 Jun 30;13(1):22. doi: 10.1038/s41368-021-00126-4. Int J Oral Sci. 2021. PMID: 34193832 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

