Systematic evaluation of levodopa-carbidopa intestinal gel patient-responder characteristics
- PMID: 29387783
- PMCID: PMC5784118
- DOI: 10.1038/s41531-017-0040-2
Systematic evaluation of levodopa-carbidopa intestinal gel patient-responder characteristics
Abstract
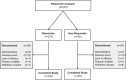
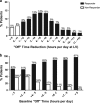
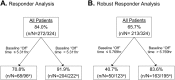
Levodopa-carbidopa intestinal gel (LCIG, carbidopa-levodopa enteral suspension in the United States) is a treatment option for advanced Parkinson's disease (PD) patients with motor fluctuations. The objective of this investigation was to identify the baseline characteristics predictive of treatment response, measured by improvement in motor symptom severity, in advanced PD patients treated with LCIG during a 54-week, open-label phase 3 study. Patients with ≥1 h improvement from baseline in "Off" time were categorized as "Responders"; whereas those with <1 h improvement, any worsening, or no post-baseline assessment were "Non-Responders". A subgroup of Responders with ≥3 h improvement in "Off" time was also examined; this subgroup was identified as "Robust Responders". Baseline demographics and disease characteristics were analyzed and their predictive relationship to change from baseline in normalized "Off" time was assessed. Out of the 324 patients included in the analysis, 272 (84.0%) were categorized as Responders and 52 (16.0%) were Non-Responders. A majority of patients (65.7%) had ≥3 h improvement in "Off" time. In general, baseline characteristics were similar between Non-responders, Responders, and the subgroup of Robust Responders. A conditional tree-structured regression analysis identified baseline "Off" time as the only factor that had significant effect on Responder and Robust Responder status. The safety profile of LCIG was similar between patient groups. Overall, this analysis showed that 84% of LCIG-treated advanced PD patients had ≥1 h improvement in "Off" time and the number-needed-to-treat to observe one patient responder was 1.19 patients. Notably, Responders and Robust Responders to LCIG were observed across the range of baseline demographics and clinical characteristics examined.
Conflict of interest statement
D. G. S. is a member of the faculty of the University of Alabama at Birmingham and is supported by endowment and University funds. D. G. S. is an investigator in studies funded by Abbvie, Inc., the American Parkinson Disease Association, the Michael J. Fox Foundation for Parkinson Research, Alabama Department of Commerce, and NIH grants P01NS087997, P20NS087997, R25NS079188, P2CHD086851, and P30NS047466. He has a clinical practice and is compensated for these activities through the University of Alabama Health Services Foundation. In addition, since 1st January 2016 he has served as a consultant for or received honoraria from Serina Therapeutics, Abbvie, Voyager Therapeutics, the Michael J. Fox Foundation for Parkinson Research, The International Parkinson Disease and Movement Disorder Society, the National Institutes of Health, The American Institute for Biological Sciences, Rush University, Huntsville Hospital, UCSD, Voyager Therapeutics, and he has received royalties for publications from McGraw Hill, Inc. J. T. B. served as a consultant and/or scientific advisor for AbbVie Inc., Auspex, Teva, Lundbeck, Chronos Therapeutics, Neurocrine, and Medical Education Resources. He has received research support from the Michael J. Fox Foundation for Parkinson Research, NIH/NINDS, Auspex, Biotie, Cure Huntington’s Disease Initiative, Vaccinex, Teva, AbbVie Inc., NeuroDerm, and Roche. P. O. was a study investigator and has received compensations for consultancy and speaker related activities from AbbVie, Britannia, Boehringer-Ingelheim, Nordic Infucare, UCB, and Zambon. P. O. has received royalties from Uni-Med Verlag. W. Z. R. and J. Z. are employees of AbbVie and hold AbbVie stock and/or stock options. K. C. is a former employee of AbbVie and and holds AbbVie stock and/or stock options.
Figures
Similar articles
-
Levodopa-Carbidopa Intestinal Gel Monotherapy: GLORIA Registry Demographics, Efficacy, and Safety.J Parkinsons Dis. 2019;9(3):531-541. doi: 10.3233/JPD-191605. J Parkinsons Dis. 2019. PMID: 31282424 Free PMC article.
-
Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson's disease patients.J Parkinsons Dis. 2015;5(1):165-74. doi: 10.3233/JPD-140456. J Parkinsons Dis. 2015. PMID: 25588353 Clinical Trial.
-
Effect of Levodopa-carbidopa Intestinal Gel on Non-motor Symptoms in Patients with Advanced Parkinson's Disease.Mov Disord Clin Pract. 2017 Nov-Dec;4(6):829-837. doi: 10.1002/mdc3.12526. Epub 2017 Sep 20. Mov Disord Clin Pract. 2017. PMID: 29242809 Free PMC article.
-
Levodopa-carbidopa enteral suspension in advanced Parkinson's disease: clinical evidence and experience.Ther Adv Neurol Disord. 2017 Mar;10(3):171-187. doi: 10.1177/1756285616681280. Epub 2016 Dec 1. Ther Adv Neurol Disord. 2017. PMID: 28344656 Free PMC article. Review.
-
Can suitable candidates for levodopa/carbidopa intestinal gel therapy be identified using current evidence?eNeurologicalSci. 2017 Jul 2;8:44-53. doi: 10.1016/j.ensci.2017.06.004. eCollection 2017 Sep. eNeurologicalSci. 2017. PMID: 29260038 Free PMC article. Review.
Cited by
-
Sustained response in early responders to safinamide in patients with Parkinson's disease and motor fluctuations: A post hoc analysis of the SETTLE study.Front Neurol. 2023 Mar 27;14:1147008. doi: 10.3389/fneur.2023.1147008. eCollection 2023. Front Neurol. 2023. PMID: 37051060 Free PMC article.
-
A post hoc comparison of levodopa-carbidopa intestinal gel daytime monotherapy vs polytherapy safety and efficacy in patients with advanced Parkinson's disease: Results from 6 phase 3/3b open-label studies.Clin Park Relat Disord. 2019 Dec 11;2:25-34. doi: 10.1016/j.prdoa.2019.12.001. eCollection 2020. Clin Park Relat Disord. 2019. PMID: 34316616 Free PMC article.
-
A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson's Disease: Post-Hoc Analysis of Motor Outcomes.J Clin Med. 2023 Apr 13;12(8):2846. doi: 10.3390/jcm12082846. J Clin Med. 2023. PMID: 37109183 Free PMC article.
-
Intrajejunal Infusion of Levodopa/Carbidopa for Advanced Parkinson's Disease: A Systematic Review.Mov Disord. 2021 Aug;36(8):1759-1771. doi: 10.1002/mds.28595. Epub 2021 Apr 25. Mov Disord. 2021. PMID: 33899262 Free PMC article.
-
Predictors of Response for "Off" Time Improvement With Levodopa-Carbidopa Intestinal Gel Treatment: An Analysis of the GLORIA Registry.Front Neurol. 2020 Jun 19;11:419. doi: 10.3389/fneur.2020.00419. eCollection 2020. Front Neurol. 2020. PMID: 32636792 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

