Retinoic acid signaling and neurogenic niche regulation in the developing peripheral nervous system of the cephalochordate amphioxus
- PMID: 29387904
- PMCID: PMC11105557
- DOI: 10.1007/s00018-017-2734-3
Retinoic acid signaling and neurogenic niche regulation in the developing peripheral nervous system of the cephalochordate amphioxus
Abstract
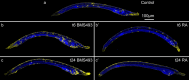
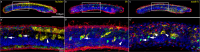
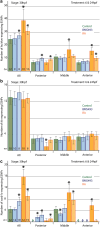
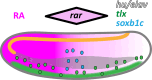
The retinoic acid (RA) signaling pathway regulates axial patterning and neurogenesis in the developing central nervous system (CNS) of chordates, but little is known about its roles during peripheral nervous system (PNS) formation and about how these roles might have evolved. This study assesses the requirement of RA signaling for establishing a functional PNS in the cephalochordate amphioxus, the best available stand-in for the ancestral chordate condition. Pharmacological manipulation of RA signaling levels during embryogenesis reduces the ability of amphioxus larvae to respond to sensory stimulation and alters the number and distribution of ectodermal sensory neurons (ESNs) in a stage- and context-dependent manner. Using gene expression assays combined with immunohistochemistry, we show that this is because RA signaling specifically acts on a small population of soxb1c-expressing ESN progenitors, which form a neurogenic niche in the trunk ectoderm, to modulate ESN production during elongation of the larval body. Our findings reveal an important role for RA signaling in regulating neurogenic niche activity in the larval amphioxus PNS. Although only few studies have addressed this issue so far, comparable RA signaling functions have been reported for neurogenic niches in the CNS and in certain neurogenic placode derivatives of vertebrates. Accordingly, the here-described mechanism is likely a conserved feature of chordate embryonic and adult neural development.
Keywords: Evolution of development; Lancelet; Neural stem cells; Retinoid pathway; Sensory functions.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
BMP and Delta/Notch signaling control the development of amphioxus epidermal sensory neurons: insights into the evolution of the peripheral sensory system.Development. 2012 Jun;139(11):2020-30. doi: 10.1242/dev.073833. Epub 2012 Apr 25. Development. 2012. PMID: 22535413
-
Roles of Retinoic Acid Signaling in Shaping the Neuronal Architecture of the Developing Amphioxus Nervous System.Mol Neurobiol. 2018 Jun;55(6):5210-5229. doi: 10.1007/s12035-017-0727-8. Epub 2017 Sep 5. Mol Neurobiol. 2018. PMID: 28875454
-
CYP26 function is required for the tissue-specific modulation of retinoic acid signaling during amphioxus development.Int J Dev Biol. 2017;61(10-11-12):733-747. doi: 10.1387/ijdb.170227ms. Int J Dev Biol. 2017. PMID: 29319120
-
From so simple a beginning - what amphioxus can teach us about placode evolution.Int J Dev Biol. 2017;61(10-11-12):633-648. doi: 10.1387/ijdb.170127gs. Int J Dev Biol. 2017. PMID: 29319112 Review.
-
Origin and evolution of the chordate central nervous system: insights from amphioxus genoarchitecture.Int J Dev Biol. 2017;61(10-11-12):655-664. doi: 10.1387/ijdb.170258jg. Int J Dev Biol. 2017. PMID: 29319114 Review.
Cited by
-
Retinoic Acid and POU Genes in Developing Amphioxus: A Focus on Neural Development.Cells. 2023 Feb 14;12(4):614. doi: 10.3390/cells12040614. Cells. 2023. PMID: 36831281 Free PMC article.
-
An Updated Staging System for Cephalochordate Development: One Table Suits Them All.Front Cell Dev Biol. 2021 May 20;9:668006. doi: 10.3389/fcell.2021.668006. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095136 Free PMC article.
-
The evolutionary origins of the vertebrate olfactory system.Open Biol. 2020 Dec;10(12):200330. doi: 10.1098/rsob.200330. Epub 2020 Dec 23. Open Biol. 2020. PMID: 33352063 Free PMC article. Review.
-
Highly distinct genetic programs for peripheral nervous system formation in chordates.BMC Biol. 2022 Jun 27;20(1):152. doi: 10.1186/s12915-022-01355-7. BMC Biol. 2022. PMID: 35761237 Free PMC article.
-
Insights into vitamin A in bladder cancer, lack of attention to gut microbiota?Front Immunol. 2023 Aug 29;14:1252616. doi: 10.3389/fimmu.2023.1252616. eCollection 2023. Front Immunol. 2023. PMID: 37711628 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

