Rotavirus Vaccines: Effectiveness, Safety, and Future Directions
- PMID: 29388076
- PMCID: PMC5955791
- DOI: 10.1007/s40272-018-0283-3
Rotavirus Vaccines: Effectiveness, Safety, and Future Directions
Abstract
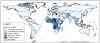
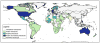
Rotavirus is the leading cause of diarrheal death among children < 5 years old worldwide, estimated to have caused ~ 215,000 deaths in 2013. Prior to rotavirus vaccine implementation, > 65% of children had at least one rotavirus diarrhea illness by 5 years of age and rotavirus accounted for > 40% of all-cause diarrhea hospitalizations globally. Two live, oral rotavirus vaccines have been implemented nationally in > 100 countries since 2006 and their use has substantially reduced the burden of severe diarrheal illness in all settings. Vaccine efficacy and effectiveness estimates suggest there is a gradient in vaccine performance between low child-mortality countries (> 90%) and medium and high child-mortality countries (57-75%). Additionally, an increased risk of intussusception (~ 1-6 per 100,000 vaccinated infants) following vaccination has been documented in some countries, but this is outweighed by the large benefits of vaccination. Two additional live, oral rotavirus vaccines were recently licensed and these have improved on some programmatic limitations of earlier vaccines, such as heat stability, cost, and cold-chain footprint. Non-replicating rotavirus vaccines that are parenterally administered are in clinical testing, and these have the potential to reduce the performance differential and safety concerns associated with live oral rotavirus vaccines.
Figures
References
-
- Causes of child mortality, by country, 2000–2010. 2017 Aug 29; ]; Available from: http://www.who.int/gho/child_health/mortality/mortality_causes_text/en/
-
- Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991 May-Jun;13(3):448–61. - PubMed
-
- Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–6. - PubMed
-
- Fischer TK, Valentiner-Branth P, Steinsland H, Perch M, Santos G, Aaby P, et al. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, west Africa. J Infect Dis. 2002 Sep 1;186(5):593–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical