Vaccines for preventing influenza in healthy children
- PMID: 29388195
- PMCID: PMC6491174
- DOI: 10.1002/14651858.CD004879.pub5
Vaccines for preventing influenza in healthy children
Abstract
Background: The consequences of influenza in children and adults are mainly absenteeism from school and work. However, the risk of complications is greatest in children and people over 65 years of age. This is an update of a review published in 2011. Future updates of this review will be made only when new trials or vaccines become available. Observational data included in previous versions of the review have been retained for historical reasons but have not been updated because of their lack of influence on the review conclusions.
Objectives: To assess the effects (efficacy, effectiveness, and harm) of vaccines against influenza in healthy children.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 12), which includes the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE (1966 to 31 December 2016), Embase (1974 to 31 December 2016), WHO International Clinical Trials Registry Platform (ICTRP; 1 July 2017), and ClinicalTrials.gov (1 July 2017).
Selection criteria: Randomised controlled trials comparing influenza vaccines with placebo or no intervention in naturally occurring influenza in healthy children under 16 years. Previous versions of this review included 19 cohort and 11 case-control studies. We are no longer updating the searches for these study designs but have retained the observational studies for historical purposes.
Data collection and analysis: Review authors independently assessed risk of bias and extracted data. We used GRADE to rate the certainty of evidence for the key outcomes of influenza, influenza-like illness (ILI), complications (hospitalisation, ear infection), and adverse events. Due to variation in control group risks for influenza and ILI, absolute effects are reported as the median control group risk, and numbers needed to vaccinate (NNVs) are reported accordingly. For other outcomes aggregate control group risks are used.
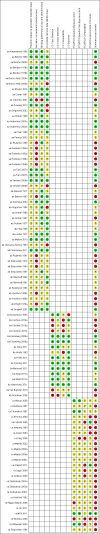
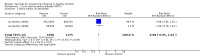
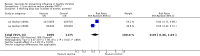
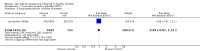
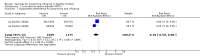
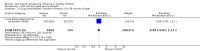
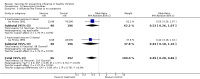
Main results: We included 41 clinical trials (> 200,000 children). Most of the studies were conducted in children over the age of two and compared live attenuated or inactivated vaccines with placebo or no vaccine. Studies were conducted over single influenza seasons in the USA, Western Europe, Russia, and Bangladesh between 1984 and 2013. Restricting analyses to studies at low risk of bias showed that influenza and otitis media were the only outcomes where the impact of bias was negligible. Variability in study design and reporting impeded meta-analysis of harms outcomes.Live attenuated vaccinesCompared with placebo or do nothing, live attenuated influenza vaccines probably reduce the risk of influenza infection in children aged 3 to 16 years from 18% to 4% (risk ratio (RR) 0.22, 95% confidence interval (CI) 0.11 to 0.41; 7718 children; moderate-certainty evidence), and they may reduce ILI by a smaller degree, from 17% to 12% (RR 0.69, 95% CI 0.60 to 0.80; 124,606 children; low-certainty evidence). Seven children would need to be vaccinated to prevent one case of influenza, and 20 children would need to be vaccinated to prevent one child experiencing an ILI. Acute otitis media is probably similar following vaccine or placebo during seasonal influenza, but this result comes from a single study with particularly high rates of acute otitis media (RR 0.98, 95% CI 0.95 to 1.01; moderate-certainty evidence). There was insufficient information available to determine the effect of vaccines on school absenteeism due to very low-certainty evidence from one study. Vaccinating children may lead to fewer parents taking time off work, although the CI includes no effect (RR 0.69, 95% CI 0.46 to 1.03; low-certainty evidence). Data on the most serious consequences of influenza complications leading to hospitalisation were not available. Data from four studies measuring fever following vaccination varied considerably, from 0.16% to 15% in children who had live vaccines, while in the placebo groups the proportions ranged from 0.71% to 22% (very low-certainty evidence). Data on nausea were not reported.Inactivated vaccinesCompared with placebo or no vaccination, inactivated vaccines reduce the risk of influenza in children aged 2 to 16 years from 30% to 11% (RR 0.36, 95% CI 0.28 to 0.48; 1628 children; high-certainty evidence), and they probably reduce ILI from 28% to 20% (RR 0.72, 95% CI 0.65 to 0.79; 19,044 children; moderate-certainty evidence). Five children would need to be vaccinated to prevent one case of influenza, and 12 children would need to be vaccinated to avoid one case of ILI. The risk of otitis media is probably similar between vaccinated children and unvaccinated children (31% versus 27%), although the CI does not exclude a meaningful increase in otitis media following vaccination (RR 1.15, 95% CI 0.95 to 1.40; 884 participants; moderate-certainty evidence). There was insufficient information available to determine the effect of vaccines on school absenteeism due to very low-certainty evidence from one study. We identified no data on parental working time lost, hospitalisation, fever, or nausea.We found limited evidence on secondary cases, requirement for treatment of lower respiratory tract disease, and drug prescriptions. One brand of monovalent pandemic vaccine was associated with a sudden loss of muscle tone triggered by the experience of an intense emotion (cataplexy) and a sleep disorder (narcolepsy) in children. Evidence of serious harms (such as febrile fits) was sparse.
Authors' conclusions: In children aged between 3 and 16 years, live influenza vaccines probably reduce influenza (moderate-certainty evidence) and may reduce ILI (low-certainty evidence) over a single influenza season. In this population inactivated vaccines also reduce influenza (high-certainty evidence) and may reduce ILI (low-certainty evidence). For both vaccine types, the absolute reduction in influenza and ILI varied considerably across the study populations, making it difficult to predict how these findings translate to different settings. We found very few randomised controlled trials in children under two years of age. Adverse event data were not well described in the available studies. Standardised approaches to the definition, ascertainment, and reporting of adverse events are needed. Identification of all global cases of potential harms is beyond the scope of this review.
Conflict of interest statement
Tom Jefferson (TJ) was a recipient of a UK National Institute for Health Research grant for a Cochrane Review of neuraminidase inhibitors for influenza. In addition, TJ receives royalties from his books published by Il Pensiero Scientifico Editore, Rome and Blackwells. TJ is occasionally interviewed by market research companies about phase I or II pharmaceutical products. In 2011‐13, TJ acted as an expert witness in litigation related to the antiviral oseltamivir, in two litigation cases on potential vaccine‐related damage, and in a labour case on influenza vaccines in healthcare workers in Canada. He has acted as a consultant for Roche (1997‐99), GSK (2001‐2), Sanofi‐Synthelabo (2003), and IMS Health (2013). In 2014 he was retained as a scientific adviser to a legal team acting on oseltamivir. TJ has a potential financial conflict of interest in the drug oseltamivir. In 2014‐16, TJ was a member of three advisory boards for Boerhinger Ingelheim. He is holder of a Cochrane Methods Innovations Fund grant to develop guidance on the use of regulatory data in Cochrane Reviews. TJ was a member of an independent data monitoring committee for a Sanofi Pasteur clinical trial on an influenza vaccine. Between 1994 and 2013, TJ was the co‐ordinator of the Cochrane Vaccines Field. TJ is a cosignatory of the Nordic Cochrane Centre Complaint to the European Medicines Agency (EMA) over maladministration at the EMA in relation to the investigation of alleged harms of human papillomavirus vaccines and consequent complaints to the European Ombudsman.
Alessandro Rivetti: none known.
Carlo Di Pietrantonj: none known.
Vittorio Demicheli: none known.
Eliana Ferroni: none known.
Figures





























Update of
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD004879. doi: 10.1002/14651858.CD004879.pub4. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2018 Feb 01;2:CD004879. doi: 10.1002/14651858.CD004879.pub5. PMID: 22895945 Free PMC article. Updated.
References
References to studies included in this review
aa Alexandrova 1986 {published data only}
-
- Alexandrova GI, Budilovsky GN, Koval TA, Polezhaev FI, Garmashova LM, Ghendon YuZ, et al. Study of live recombinant cold‐adapted influenza bivalent vaccine of type A for use in children: an epidemiological control trial. Vaccine 1986;4(2):114‐8. - PubMed
aa Belshe 1998 {published data only}
-
- Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine in children. New England Journal of Medicine 1998;338(20):1405‐12. - PubMed
-
- Piedra PA, Yan L, Kotloff K, Zangwill K, Bernstein DI, King J, et al. Safety of the trivalent, cold‐adapted influenza vaccine in preschool‐aged children. Pediatrics 2002;110(4):662‐72. - PubMed
aa Belshe 2000a {published data only}
-
- Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, et al. Efficacy of vaccination with live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. Journal of Pediatrics 2000;136(2):168‐75. - PubMed
-
- Piedra PA, Yan L, Kotloff K, Zangwill K, Bernstein DI, King J, et al. Safety of the trivalent, cold‐adapted influenza vaccine in preschool‐aged children. Pediatrics 2002;110(4):662‐72. - PubMed
aa Beutner 1979a {published data only}
-
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase‐specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. Journal of Infectious Diseases 1979;140(6):844‐50. - PubMed
aa Beutner 1979b {published data only}
-
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase‐specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. Journal of Infectious Diseases 1979;140(6):844‐50. - PubMed
aa Bracco Neto 2009a {published data only}
-
- Bracco Neto H, Farhat CK, Tregnaghi MW, Madhi SA, Razmpour A, Palladino G, et al. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine‐naive children. Pediatric Infectious Disease Journal 2009;28(5):365‐71. - PubMed
aa Bracco Neto 2009b {published data only}
-
- Bracco Neto H, Farhat CK, Tregnaghi MW, Madhi SA, Razmpour A, Palladino G, et al. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine‐naive children. Pediatric Infectious Disease Journal 2009;28(5):365‐71. - PubMed
aa Brooks 2016 {published data only}
aa Clover 1991 {published data only}
-
- Clover RD, Crawford S, Glezen WP, Taber LH, Matson CC, Couch RB. Comparison of heterotypic protection against influenza A/Taiwan/86 (H1N1) by attenuated and inactivated vaccines to A/Chile/83‐like viruses. Journal of Infectious Diseases 1991;163(2):300‐4. - PubMed
aa Colombo 2001 {published data only}
-
- Colombo C, Argiolas L, Vecchia C, Negri E, Meloni G, Meloni T. Influenza vaccine in healthy preschool children. Revue d'Epidémiologie et de Santé Publique 2001;49(2):157‐62. - PubMed
aa Cowling 2012 {published data only}
-
- Cowling BJ, Ng S, Ma ES, Fang VJ, So HC, Wai W, et al. Protective efficacy against pandemic influenza of seasonal influenza vaccination in children in Hong Kong: a randomized controlled trial. Clinical Infectious Diseases 2012;55(5):695‐702. - PubMed
aa Grigor'eva 2002 {published data only}
-
- Grigor'eva EP, Desheva I, Donina SA, Naikhin AN, Rekstin AR, Barantseva IB, et al. The comparative characteristics of the safety, immunogenic activity and prophylactic potency of the adult and children types of live influenza vaccine in schoolchildren aged 7‐14 years. Voprosy Virusologii 2002;47(4):24‐7. - PubMed
aa Gruber 1990 {published data only}
-
- Gruber WC, Taber LH, Glezen WP, Clover RD, Abell TD, Demmler RW, et al. Live attenuated and inactivated influenza vaccine in school‐age children. American Journal of Diseases in Children 1990;144(5):595‐600. - PubMed
aa Hoberman 2003a {published data only}
-
- Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290(12):1608‐16. - PubMed
aa Hoberman 2003b {published data only}
-
- Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290(12):1608‐16. - PubMed
aa Khan 1996 {published data only}
-
- Khan AS, Polezhaev F, Vasiljeva R, Drinevsky V, Buffington J, Gary H, et al. Comparison of US inactivated split‐virus and Russian live attenuated, cold‐adapted trivalent influenza vaccines in Russian schoolchildren. Journal of Infectious Diseases 1996;173(2):453‐6. - PubMed
aa Principi 2003 {published data only}
-
- Principi N, Esposito S, Marchisio P, Gasparini R, Crovari P. Socioeconomic impact of influenza on healthy children and their families. Pediatric Infectious Disease Journal 2003;22(Suppl 10):207‐10. - PubMed
aa Rudenko 1988 {published data only}
-
- Rudenko LG, Grigor'eva EP, Drinevskii VP, Koval' TA, Doroshenko EM. Results of a study of a live intranasal influenza vaccine in the immunization of children 3 to 15 years old. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1988;5:41‐6. - PubMed
aa Rudenko 1993a {published data only}
-
- Rudenko LG, Slepushkin AN, Monto AS, Kendal AP, Grigorieva EP, Burtseva EP, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. Journal of Infectious Diseases 1993;168(4):881‐7. - PubMed
aa Rudenko 1993b {published data only}
-
- Rudenko LG, Slepushkin AN, Monto AS, Kendal AP, Grigorieva EP, Burtseva EP, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. Journal of Infectious Diseases 1993;168(4):881‐7. - PubMed
aa Rudenko 1996a {published data only}
aa Rudenko 1996b {published data only}
-
- Rudenko LG, Vasil'eva RI, Ismagulov AT, Karagodina VI, Slepushkin AN, Doroshenko EM, et al. Prophylactic effectiveness of a live recombinant influenza type A vaccine in immunizing children aged 3‐14 years. Voprosy Virusologii 1996;41:37‐9. - PubMed
aa Tam 2007a {published data only}
-
- Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, Huang LM, et al. Efficacy and safety of a live attenuated, cold‐adapted influenza vaccine, trivalent against culture‐confirmed influenza in young children in Asia. Pediatric Infectious Disease Journal 2007;26(7):619‐28. - PubMed
aa Tam 2007b {published data only}
-
- Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, Huang LM, et al. Efficacy and safety of a live attenuated, cold‐adapted influenza vaccine, trivalent against culture‐confirmed influenza in young children in Asia. Pediatric Infectious Disease Journal 2007;26(7):619‐28. - PubMed
aa Vesikari 2006a {published data only}
-
- Vesikari T, Fleming DM, Aristegui JF, Vertruyen A, Ashkenazi S, Rappaport R, et al. Safety, efficacy, and effectiveness of cold‐adapted influenza vaccine‐trivalent against community‐acquired, culture‐confirmed influenza in young children attending day care. Pediatrics 2006;118:2298‐312. - PubMed
aa Vesikari 2006b {published data only}
-
- Vesikari T, Fleming DM, Aristegui JF, Vertruyen A, Ashkenazi S, Rappaport R, et al. Safety, efficacy, and effectiveness of cold‐adapted influenza vaccine‐trivalent against community‐acquired, culture‐confirmed influenza in young children attending day care. Pediatrics 2006;118:2298‐312. - PubMed
ab Aksenov 1971 {published data only}
-
- Aksenov VA, Selidovkin DA, Sorokina LV, Burnos AS, Sorokina AG. The effectiveness of influenza prevention with a special variation of live influenza vaccine for children in the 1969 epidemic. Voprosy Virusologii 1971;16:81‐6. - PubMed
ab Belshe 1992 {published data only}
-
- Belshe RB, Swierkosz EM, Anderson EL, Newman FK, Nugent SL, Maassab HF. Immunization of infants and young children with live attenuated trivalent cold‐recombinant influenza A H1N1, H3N2, and B vaccine. Journal of Infectious Diseases 1992;165(4):727‐32. - PubMed
ab Desheva 2002 {published data only}
-
- Desheva I, Danini GV, Grigor'eva EP, Donina SA, Kiseleva IV, Rekstin AR, et al. The investigation of the safety, genetic stability and immunogenicity of live influenza vaccine for adults in vaccination of 3‐6 year old children. Voprosy Virusologii 2002;47(4):21‐4. - PubMed
ab Grigor'eva 1994 {published data only}
-
- Grigor'eva EP, Rekstin AR, Rudenko LG, Ramirez A, Barro M, Lisovskaia KV, et al. The immunogenic properties and prophylactic efficacy of a live polyvalent influenza vaccine in children 5 to 14 years old. Voprosy Virusologii 1994;39(1):26‐9. - PubMed
ab Gruber 1996 {published data only}
-
- Gruber WC, Belshe RB, King JC, Treanor JJ, Piedra PA, Wright PF, et al. Evaluation of live attenuated influenza vaccines in children 6‐18 months of age: safety, immunogenicity, and efficacy. National Institute of Allergy and Infectious Diseases, Vaccine and Treatment Evaluation Program and the Wyeth‐Ayerst ca Influenza Vaccine Investigators Group. Journal of Infectious Diseases 1996;173(6):1313‐9. - PubMed
ab Gruber 1997 {published data only}
-
- Gruber WC, Darden PM, Still JG, Lohr J, Reed G, Wright PF. Evaluation of bivalent live attenuated influenza A vaccines in children 2 months to 3 years of age: safety, immunogenicity and dose‐response. Vaccine 1997;15(12‐3):1379‐84. - PubMed
ab Gutman 1977 {published data only}
-
- Gutman LT, Wilfert CM, Idriss ZH, Schmidt E, Andrews S, Katz SL. Single‐dose trials of monovalent A/New Jersey/76 (Hsw1N1) influenza virus vaccine in children in Durham, North Carolina. Journal of Infectious Diseases 1977;136(Suppl):575‐8. - PubMed
ab King 1998 {published data only}
-
- King JC Jr, Lagos R, Bernstein DI, Piedra PA, Kotloff K, Bryant M, et al. Safety and immunogenicity of low and high doses of trivalent live cold‐adapted influenza vaccine administered intranasally as drops or spray to healthy children. Journal of Infectious Diseases 1998;17(5):1394‐7. - PubMed
ab Levine 1977 {published data only}
-
- Levine MM, Hughes TP, Simon P, O'Donnell S, Grauel S, Levine SG. Monovalent inactivated A/New Jersey/8/76 (Hsw1N1) vaccine in healthy children aged three to five years. Journal of Infectious Diseases 1977;136(Suppl):571‐4. - PubMed
ab Mallory 2010 {published data only}
ab Obrosova‐Serova 1990 {published data only}
-
- Obrosova‐Serova NP, Slepushkin AN, Kendal AP, Harmon MW, Burtseva EI, Bebesheva NI, et al. Evaluation in children of cold‐adapted influenza B live attenuated intranasal vaccine prepared by reassortment between wild‐type B/Ann Arbor/1/86 and cold‐adapted B/Leningrad/14/55 viruses. Vaccine 1990;8(1):57‐60. - PubMed
ab Plennevaux 2011 {published data only}
-
- Plennevaux E, Blatter M, Cornish MJ, Go K, Kirby D, Wali M, et al. Influenza A (H1N1) 2009 two‐dose immunization of US children: an observer‐blinded, randomized, placebo‐controlled trial. Vaccine 2011;29(8):1569‐75. - PubMed
ab Rudenko 1991 {published data only}
-
- Rudenko LG, Ramirez A, Barro M, Gushchina MI, Armanda RE, Ermachenko TA, et al. The inoculation properties of live recombinant influenza vaccine types A and B used separately and jointly in children 3 to 14. Voprosy Virusologii 1991;36(6):472‐4. - PubMed
ab Slepushkin 1974 {published data only}
-
- Slepushkin AN, Dukova VS, Kalegaeva VA, Kagan AN, Temriuk EE. Results of studying the effectiveness of a live influenza vaccine for peroral use on preschool and schoolchildren. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1974;12:24‐9. - PubMed
ab Slepushkin 1988 {published data only}
-
- Slepushkin AN, Patriarca PA, Obrosova‐Serova NP, Harmon MW, Kupryashina LN, Cheshik SG, et al. Class‐specific antibody responses in school children vaccinated with an A/Brazil/11/78 (H1N1)‐like recombinant influenza virus prepared from the A/Leningrad/134/57 paediatric cold‐adapted donor strain. Vaccine 1988;6(1):25‐8. - PubMed
ab Slepushkin 1991 {published data only}
-
- Slepushkin AN, Obrosova‐Serova NP, Burtseva EI, Govorkova EA, Rudenko LG, Vartanian RV, et al. A comparative study of the inoculation properties of live recombinant and inactivated influenza vaccines made from strain A/Philippines/2/82 (H3N2) in 8‐ to 15‐year‐old children. Voprosy Virusologii 1991;36(5):372‐4. - PubMed
ab Steinhoff 1990 {published data only}
-
- Steinhoff MC, Halsey NA, Wilson MH, Burns BA, Samorodin RK, Fries LF, et al. Comparison of live attenuated cold‐adapted and avian‐human influenza A/Bethesda/85 (H3N2) reassortant virus vaccines in infants and children. Journal of Infectious Diseases 1990;162(2):394‐401. - PubMed
ab Steinhoff 1991 {published data only}
-
- Steinhoff MC, Halsey NA, Fries LF, Wilson MH, King J, Burns BA, et al. The A/Mallard/6750/78 avian‐human, but not the A/Ann Arbor/6/60 cold‐adapted, influenza A/Kawasaki/86 (H1N1) reassortant virus vaccine retains partial virulence for infants and children. Journal of Infectious Diseases 1991;163(5):1023‐8. - PubMed
ab Swierkosz 1994 {published data only}
-
- Swierkosz EM, Newman FK, Anderson EL, Nugent SL, Mills GB, Belshe RB. Multidose, live attenuated, cold‐recombinant, trivalent influenza vaccine in infants and young children. Journal of Infectious Diseases 1994;169(5):1121‐4. - PubMed
ab Vasil'eva 1988a {published data only}
-
- Vasil'eva RI, Merkur'eva LA, Latsenko VG, Vasil'eva AM, Shvager MM. Characteristics of the clinical and immunologic safety of inactivated influenza vaccines in children undergoing multiple immunizations. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1988;11:65‐9. - PubMed
ab Vasil'eva 1988b {published data only}
-
- Vasil'eva RI, Smirnova LA, Rudenko LG, Drinevskii VP, Tsaritsina IM. The results of state trials of inactivated influenza vaccines for children. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1988;3:49‐54. - PubMed
ab Wright 1976a {published data only}
-
- Wright PF, Sell SH, Thompson J, Karzon DT. Clinical reactions and serologic response following inactivated monovalent influenza type B vaccine in young children and infants. Journal of Pediatrics 1976;88(1):31‐5. - PubMed
ab Zangwill 2001 {published and unpublished data}
-
- Zangwill KM, Droge J, Mendelman P, Marcy SM, Partridge S, Chiu CY, et al. Prospective, randomized, placebo‐controlled evaluation of the safety and immunogenicity of three lots of intranasal trivalent influenza vaccine among young children. Pediatic Infectious Disease Journal 2001;20(8):740‐6. - PubMed
ba Anonymous 2005 {published data only}
-
- Effectiveness of vaccine against medical consultation due to laboratory‐confirmed influenza: results from a sentinel physician pilot project in British Columbia, 2004‐2005. Canada Communicable Disease Report 2005;31(18):181‐91. - PubMed
ba Cochran 2010a {published data only}
-
- Cochran LW, Black S, Klein NP, Dekker CL, Lewis E, Reingold AL. Vaccine effectiveness against laboratory‐confirmed influenza in infants: a matched case control study. Human Vaccines 2010;6(9):729‐35. - PubMed
ba Cochran 2010b {published data only}
-
- Cochran LW, Black S, Klein NP, Dekker CL, Lewis E, Reingold AL. Vaccine effectiveness against laboratory‐confirmed influenza in infants: a matched case control study. Human Vaccines 2010;23(6):729‐35. - PubMed
ba Cochran 2010c {published data only}
-
- Cochran LW, Black S, Klein NP, Dekker CL, Lewis E, Reingold AL. Vaccine effectiveness against laboratory‐confirmed influenza in infants: a matched case control study. Human Vaccines 2010;23(6):729‐35. - PubMed
ba Eisenberg 2008a {published data only}
ba Eisenberg 2008b {published data only}
ba Gilca 2011 {published data only}
-
- Gilca R, Deceuninck G, Serres G, Boulianne N, Sauvageau C, Quach C, et al. Effectiveness of pandemic H1N1 vaccine against influenza‐related hospitalization in children. Pediatrics 2011;128(5):e1084‐91. - PubMed
ba Hirota 1992 {published data only}
-
- Hirota Y, Takeshita S, Ide S, Kataoka K, Ohkubo A, Fukuyoshi S, et al. Various factors associated with the manifestation of influenza‐like illness. International Journal of Epidemiology 1992;21(3):574‐82. - PubMed
ba Kelly 2011 {published data only}
-
- Kelly H, Jacoby P, Dixon GA, Carcione D, Williams S, Moore HC, et al. Vaccine effectiveness against laboratory‐confirmed influenza in healthy young children: a case‐control study. Pediatric Infectious Disease Journal 2011;30(2):107‐11. - PubMed
ba Kissling 2011 {published data only}
ba Mahmud 2011 {published data only}
-
- Mahmud S, Hammond G, Elliott L, Hilderman T, Kurbis C, Caetano P, et al. Effectiveness of the pandemic H1N1 influenza vaccines against laboratory‐confirmed H1N1 infections: population‐based case‐control study. Vaccine 2011;29(45):7975‐81. - PubMed
ba Staat 2011a {published data only}
-
- Staat MA, Griffin MR, Donauer S, Edwards KM, Szilagyi PG, Weinberg GA, et al. Vaccine effectiveness for laboratory‐confirmed influenza in children 6‐59 months of age, 2005‐2007. Vaccine 2011;29(48):9005‐11. - PubMed
ba Staat 2011b {published data only}
-
- Staat MA, Griffin MR, Donauer S, Edwards KM, Szilagyi PG, Weinberg GA, et al. Vaccine effectiveness for laboratory‐confirmed influenza in children 6‐59 months of age, 2005‐2007. Vaccine 2011;29(48):9005‐11. - PubMed
ba Valenciano 2011 {published data only}
-
- Valenciano M, Kissling E, Cohen JM, Oroszi B, Barret AS, Rizzo C, et al. Estimates of pandemic influenza vaccine effectiveness in Europe, 2009‐2010: results of Influenza Monitoring Vaccine Effectiveness in Europe (I‐MOVE) multicentre case‐control study. PLoS Medicine 2011;8(1):e1000388. - PMC - PubMed
ba Van Buynder 2010 {published data only}
bb Goodman 2006 {published data only}
-
- Goodman MJ, Nordin JD, Harper P, Defor T, Zhou X. The safety of trivalent influenza vaccine among healthy children 6 to 24 months of age. Pediatrics 2006;117(5):821‐6. - PubMed
ca Allison 2006 {published data only}
-
- Allison MA, Daley MF, Crane LA, Barrow J, Beaty BL, Allred N, et al. Influenza vaccine effectiveness in healthy 6‐ to 21‐month‐old children during the 2003‐2004 season. Journal of Pediatrics 2006;149(6):755‐62. - PubMed
ca Burtseva 1991 {published data only}
-
- Burtseva EI, Obrosova‐Serova NP, Govorkova EA, Rudenko LG, Vartanian RV, Beliaev AL, et al. A comparative study of the protective properties of live recombinant and inactivated influenza vaccines made from strain A/Philippines/2/82 (H3N2) in 8‐ to 15‐year‐old children. Voprosy Virusologii 1991;36(5):375‐7. - PubMed
ca Chumakov 1987 {published data only}
-
- Bashliaeva ZA, Sumarokov AA, Nefedova LA, Iaroshevskaia II, Ozeretskovskaia NA. Basic results of a committee trial of the new vaccine Grippovac SE‐AZh. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1986;2:49‐54. - PubMed
-
- Chumakov MP, Boiko VM, Malyshkina LP, Mel'nikova SK, Rodin VI. Results of coded trials of the activity of the trivalent subunit influenza vaccine Grippovak in Moscow kindergartens in December 1983 through the 1st quarter of 1984. Voprosy Virusologii 1987;32(2):175‐83. - PubMed
ca El'shina 2000 {published data only}
-
- El'shina GA, Gorbunov MA, Bektimirov TA, Lonskaia NI, Pavlova LI, Nikul'shin AA, et al. The evaluation of the reactogenicity, harmlessness and prophylactic efficacy of Grippol trivalent polymer‐subunit influenza vaccine administered to schoolchildren. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2000;2:50‐4. - PubMed
ca Fujieda 2006 {published data only}
-
- Fujieda M, Maeda A, Kondo K, Kaji M, Hirota Y. Inactivated influenza vaccine effectiveness in children under 6 years of age during the 2002‐2003 season. Vaccine 2006;24(7):957‐63. - PubMed
ca Jianping 1999 {published data only}
-
- Jianping H, Xin F, Changshun L, Bo Z, Linxiu G, Wei X, et al. Assessment of effectiveness of Vaxigrip. Vaccine 1999;17(Suppl 1):57‐8. - PubMed
ca Kawai 2003 {published data only}
-
- Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, Tsuchimoto T, et al. A prospective, Internet‐based study of the effectiveness and safety of influenza vaccination in the 2001‐2002 influenza season. Vaccine 2003;21(31):4507‐13. - PubMed
ca King 2006 {published data only}
-
- King JC Jr, Stoddard JJ, Gaglani MJ, Moore KA, Magder L, McClure E, et al. Effectiveness of school‐based influenza vaccination. New England Journal of Medicine 2006;355:2523‐32. - PubMed
ca Maeda 2002 {published data only}
-
- Maeda T, Shintani Y, Miyamoto H, Kawagoe H, Nakano K, Nishiyama A, et al. Prophylactic effect of inactivated influenza vaccine on young children. Pediatrics International 2002;44(1):43‐6. - PubMed
ca Maeda 2004a {published data only}
-
- Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y. Failure of inactivated influenza A vaccine to protect healthy children aged 6‐24 months. Pediatrics International 2004;46(2):122‐5. - PubMed
ca Maeda 2004b {published data only}
-
- Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y. Failure of inactivated influenza A vaccine to protect healthy children aged 6‐24 months. Pediatrics International 2004;46(2):122‐5. - PubMed
ca Maeda 2004c {published data only}
-
- Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y. Failure of inactivated influenza A vaccine to protect healthy children aged 6‐24 months. Pediatrics International 2004;46(2):122‐5. - PubMed
ca Ortqvist 2011 {published data only}
-
- Ortqvist A, Berggren I, Insulander M, Jong B, Svenungsson B. Effectiveness of an adjuvanted monovalent vaccine against the 2009 pandemic strain of influenza A (H1N1)v, in Stockholm County, Sweden. Clinical Infectious Diseases 2011;52(10):1203‐11. - PubMed
ca Ozgur 2006 {published data only}
-
- Ozgur SK, Beyazova U, Kemaloglu YK, Maral I, Sahin F, Camurdan AD, et al. Effectiveness of inactivated influenza vaccine for prevention of otitis media in children. Pediatric Infectious Disease Journal 2006;25(5):401‐4. - PubMed
ca Salleras 2006 {published data only}
-
- Salleras L, Dominguez A, Pumarola T, Prat A, Marcos MA, Garrido P, et al. Effectiveness of virosomal subunit influenza vaccine in preventing influenza‐related illnesses and its social and economic consequences in children aged 3‐14 years: a prospective cohort study. Vaccine 2006;24(44‐6):6638‐42. - PubMed
ca Slobodniuk 2002a {published data only}
-
- Slobodniuk AV, Romanenko VV, Utnitskaia OS, Motus TM, Pereverzev AV. Influence of multiplicity of immunizations of children with inactivated influenza vaccine on immune response and the effectiveness of protection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2002;4:36‐9. - PubMed
ca Slobodniuk 2002b {published data only}
-
- Slobodniuk AV, Romanenko VV, Utnitskaia OS, Motus TM, Pereverzev AV. Influence of multiplicity of immunizations of children with inactivated influenza vaccine on immune response and the effectiveness of protection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2002;4:36‐9. - PubMed
ca Slobodniuk 2002c {published data only}
-
- Slobodniuk AV, Romanenko VV, Utnitskaia OS, Motus TM, Pereverzev AV. Influence of multiplicity of immunizations of children with inactivated influenza vaccine on immune response and the effectiveness of protection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2002;4:36‐9. - PubMed
ca Vasil'eva 1982 {published data only}
-
- Vasil'eva RI, Osidak LV, Bichurina MA, Mukhina LP, Chudina LV. Evaluation of the safety, reactogenicity and antigenic activity of inactivated chromatographic influenza vaccine in school children. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1982;4:96‐8. - PubMed
ca Wiggs‐Stayner 2006 {published data only}
-
- Wiggs‐Stayner KS, Purdy TR, Go GN, McLaughlin NC, Tryzynka PS, Sines JR, et al. The impact of mass school immunization on school attendance. Journal of School of Nursing 2006;22(4):219‐22. - PubMed
ca Yin 2011 {published data only}
cb MPA 2011 {unpublished data only}
-
- MPA. Occurrence of narcolepsy with cataplexy among children and adolescents in relation to the H1N1 pandemic and Pandemrix vaccinations – results of a case inventory study by the MPA in Sweden during 2009–2010. www.lakemedelsverket.se/upload/nyheter/2011/Fallinventeringsrapport pandermrix 110630.pdf (accessed 10 January 2012).
cb Nicholls 2004 {published and unpublished data}
-
- Nicholls S, Carroll K, Crofts J, Ben‐Eliezer E, Paul J, Zambon M, et al. Outbreak of influenza A (H3N2) in a highly‐vaccinated religious community: a retrospective cohort study. Communicable Disease and Public Health 2004;7:272‐7. - PubMed
cb Ritzwoller 2005 {published data only}
-
- Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003‐2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics 2005;116(1):153‐9. - PubMed
cb Slepushkin 1994 {published data only}
-
- Slepushkin AN, Rudenko LG, Kendal AP, Monto AS, Beliaev AL, Burtseva EI, et al. A comparative study of live and inactivated influenza vaccines: the organization of the observation and the results of a study of their reactogenicity and immunogenicity. Voprosy Virusologii 1994;39:129‐31. - PubMed
References to studies excluded from this review
Ambrose 2011 {published data only}
Ambrose 2014 {published data only}
-
- Ambrose CS, Wu X, Caspard H, Belshe RB. Efficacy of live attenuated influenza vaccine against influenza illness in children as a function of illness severity. Vaccine 2014;32(43):5546‐8. - PubMed
Anderson 1992 {published data only}
Anonymous 2003 {published data only}
-
- FluMist: an intranasal live influenza vaccine. Medical Letter on Drugs and Therapeutics 2003;45(1163):65‐6. - PubMed
Beare 1968 {published data only}
-
- Beare AS, Hobson D, Reed SE, Tyrrell DA. A comparison of live and killed influenza‐virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet 1968;2(7565):418‐22. - PubMed
Belshe 2000b {published data only}
-
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine. Journal of Infectious Diseases 2000;181(3):1133‐7. - PubMed
Belshe 2000c {published data only}
-
- Belshe RB, Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatric Infectious Disease Journal 2000;19(Suppl 5):66‐71. - PubMed
Belshe 2008 {published data only}
-
- Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2‐7 years of age. Vaccine 2008;26(Suppl 4):D10‐6. - PubMed
Bergen 2004 {published data only}
-
- Bergen R, Black S, Shinefield H, Lewis E, Ray P, Hansen J, et al. Safety of cold‐adapted live attenuated influenza vaccine in a large cohort of children and adolescents. Pediatric Infectious Disease Journal 2004;23(2):138‐44. - PubMed
Betts 1977 {published data only}
-
- Betts RF, Douglas RG Jr, Roth FK, Little JW 3rd. Efficacy of live attenuated influenza A/Scotland/74 (H3N2) virus vaccine against challenge with influenza A/Victoria/3/75 (H3N2) virus. Journal of Infectious Diseases 1977;136:746‐53. - PubMed
Beutner 1976 {published data only}
-
- Beutner KR, Rizzone C, DeMello S, Ogra PL. Clinical evaluation of neuraminidase monospecific (HEqN2) recombinant influenza vaccine in children. Developments in Biological Standardization 1976;33:162‐70. - PubMed
Bichurina 1982 {published data only}
-
- Bichurina MA, Polianskaia NI, Zhebrun AB, Rozaeva NR, Mukhina LP. Preliminary results of a trial of a highly purified inactivated chromatographic influenza vaccine for children. Trudy Instituta Imeni Pastera 1982;58:14‐6. - PubMed
Block 2011 {published data only}
-
- Block SL, Yi T, Sheldon E, Dubovsky F, Falloon J. A randomized, double‐blind noninferiority study of quadrivalent live attenuated influenza vaccine in adults. Vaccine 2011;29(50):9391‐7. - PubMed
Boyce 1999 {published data only}
-
- Boyce TG, Gruber WC, Coleman‐Dockery SD, Sannella EC, Reed GW, Wolff M, et al. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine 1999;18(1‐2):82‐8. - PubMed
Boyce 2000 {published data only}
-
- Boyce TG, Hsu HH, Sannella EC, Coleman‐Dockery SD, Baylis E, Zhu Y. Safety and immunogenicity of adjuvanted and unadjuvanted subunit influenza vaccines administered intranasally to healthy adults. Vaccine 2000;19(2‐3):217‐26. - PubMed
Boyer 1977 {published data only}
-
- Boyer KM, Cherry JD, Welliver RC, Deseda‐Tous J, Zahradnik JM, Dudley JP, et al. Clinical trials with inactivated monovalent (A/New Jersey/76) and bivalent (A/New Jersey/76‐A/Victoria/75) influenza vaccines in Los Angeles children. Journal of Infectious Diseases 1977;136(Suppl):661‐4. - PubMed
Cakir 2012 {published data only}
-
- Çakir BC, Beyazova U, Kemaloğlu YK, Özkan S, Gündüz B, Özdek A. Effectiveness of pandemic influenza A/H1N1 vaccine for prevention of otitis media in children. European Journal of Pediatrics 2012;171(11):1667‐71. - PubMed
Chow 1979 {published data only}
Clements 1995 {published data only}
-
- Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6‐ to 30‐month‐old children in day care. Archives of Pediatric and Adolescent Medicine 1995;149(10):1113‐7. - PubMed
Coles 1992 {published data only}
-
- Coles FB, Balzano GJ, Morse DL. An outbreak of influenza A (H3N2) in a well immunized nursing home population. Journal of the American Geriatrics Society 1992;40(6):589‐92. - PubMed
Cowling 2014 {published data only}
-
- Cowling BJ, Perera RA, Fang VJ, Chan KH, Wai W, So HC, et al. Incidence of influenza virus infections in children in Hong Kong in a 3‐year randomized placebo‐controlled vaccine study, 2009‐2012. Clinical Infectious Diseases 2014;59(4):517‐24. - PubMed
Daubeney 1997 {published data only}
-
- Daubeney P, Taylor CJ, McGaw J, Brown EM, Ghosal S, Keeton BR, et al. Immunogenicity and tolerability of a trivalent influenza subunit vaccine (Influvac) in high‐risk children aged 6 months to 4 years. British Journal of Clinical Practice 1997;51(2):87‐90. - PubMed
Donatelli 1998 {published data only}
-
- Donatelli I, Zannolli R, Fuiano L, Biasio LR. Influenza vaccine in immunogenically naive healthy infants. European Journal of Pediatrics 1998;157(11):949‐50. - PubMed
Eddy 1970 {published data only}
-
- Eddy TS, Davies NA. The effect of vaccine on a closed epidemic of Hong Kong influenza. South African Medical Journal 1970;44(8):214‐6. - PubMed
Edwards 1994 {published data only}
-
- Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold‐adapted and inactivated vaccines for the prevention of influenza A disease. Journal of Infectious Diseases 1994;169(1):68‐76. - PubMed
El'shina 1998 {published data only}
-
- El'shina GA, Gorbunov MA, Shervarli VI, Lonskaia NI, Pavlova LI, Khaitov RM, et al. Evaluation of the effectiveness of influenza trivalent polymer subunit vaccine "Grippol". Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1998;3:40‐3. - PubMed
Feldman 1985 {published data only}
-
- Feldman S, Wright PF, Webster RG, Roberson PK, Mahoney J, Thompson J, et al. Use of influenza A virus vaccines in seronegative children: live cold‐adapted versus inactivated whole virus. Journal of Infectious Diseases 1985;152(6):1212‐8. - PubMed
Foy 1981 {published data only}
-
- Foy HM, Cooney MK, Allan ID, Frost F, Blumhagen JM, Fox JP. Influenza B virus vaccines in children and adults: adverse reactions, immune response, and observations in the field. Journal of Infectious Diseases 1981;143(5):700‐6. - PubMed
France 2004 {published data only}
-
- France EK, Glanz JM, Xu S, Davis RL, Black SB, Shinefield HR, et al. Safety of the trivalent inactivated influenza vaccine among children: a population‐based study. Archives of Pediatric and Adolescent Medicine 2004;158:1031‐6. - PubMed
Fujieda 2008 {published data only}
-
- Fujieda M, Maeda A, Kondo K, Fukushima W, Ohfuji S, Kaji M, et al. Influenza vaccine effectiveness and confounding factors among young children. Vaccine 2008;26(50):6481‐5. - PubMed
Gaglani 2004 {published data only}
-
- Gaglani MJ, Piedra PA, Herschler GB, Griffith ME, Kozinetz CA, Riggs MW, et al. Direct and total effectiveness of the intranasal, live‐attenuated, trivalent cold‐adapted influenza virus vaccine against the 2000‐2001 influenza A(H1N1) and B epidemic in healthy children. Archives of Pediatric and Adolescent Medicine 2004;158(1):65‐73. - PubMed
Gendon 2004 {published data only}
-
- Gendon IuZ, Kaira AN, El'shina GA, Akhmadulina RR. Total immunization of children against influenza decreases morbidity in a number of diseases among elderly persons during influenza epidemic. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2004;5:62‐7. - PubMed
Glezen 2001 {published data only}
-
- Glezen WP. Effectiveness of influenza vaccination of day care children in reducing influenza‐related morbidity among household contacts. Journal of Pediatrics 2001;138:782. - PubMed
Groothuis 1994 {published data only}
-
- Groothuis JR, Lehr MV, Levin MJ. Safety and immunogenicity of a purified haemagglutinin antigen in very young high‐risk children. Vaccine 1994;12(2):139‐41. - PubMed
Groothuis 1998 {published data only}
-
- Groothuis JR, King SJ, Hogerman DA, Paradiso PR, Simoes EA. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP‐2) vaccine in seropositive children with bronchopulmonary dysplasia. Journal of Infectious Diseases 1998;177(2):467‐9. - PubMed
Gross 1977a {published data only}
-
- Gross PA. Reactogenicity and immunogenicity of bivalent influenza vaccine in one‐ and two‐dose trials in children: a summary. Journal of Infectious Diseases 1977;136(Suppl):616‐25. - PubMed
Gross 1977b {published data only}
-
- Gross PA, Ennis FA, Gaerlan PF, Denson LJ, Denning CR, Schiffman D. A controlled double‐blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole‐virus and split‐product influenza vaccines in children. Journal of Infectious Diseases 1977;136(5):623‐32. - PubMed
Gross 1982 {published data only}
-
- Gross PA, Quinnan GV, Gaerlan PF, Denning CR, Davis A, Lazicki M, et al. Potential for single high‐dose influenza immunization in unprimed children. Pediatrics 1982;70(6):982‐6. - PubMed
Gruber 1993 {published data only}
-
- Gruber WC, Kirschner K, Tollefson S, Thompson J, Reed G, Edwards KM, et al. Comparison of monovalent and trivalent live attenuated influenza vaccines in young children. Journal of Infectious Diseases 1993;168(1):53‐60. - PubMed
Haba‐Rubio 2011 {published data only}
-
- Haba‐Rubio J, Rossetti AO, Tafti M, Heinzer R. Narcolepsy with cataplexy associated with H1N1 vaccination [Narcolepsie avec cataplexie a pres vaccination anti‐H1N1]. Revista de Neurologia 2011;167(8‐9):563‐6. - PubMed
Halperin 2002 {published data only}
-
- Halperin SA, Smith B, Mabrouk T, Germain M, Trepanier P, Hassell T, et al. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture‐derived influenza vaccine in healthy adults, seniors, and children. Vaccine 2002;20(7‐8):1240‐7. - PubMed
Hambidge 2006 {published data only}
-
- Hambidge SJ, Glanz JM, France EK. Safety of inactivated influenza vaccine in children 6 to 23 months old. JAMA 2006;296:1990‐7. - PubMed
Hatch 1956 {published data only}
Heikkinen 2003 {published data only}
-
- Heikkinen T, Ziegler T, Peltola V, Lehtinen P, Toikka P, Lintu M, et al. Incidence of influenza in Finnish children. Pediatic Infectious Disease Journal 2003;22(Suppl 10):204‐6. - PubMed
Hoskins 1973 {published data only}
-
- Hoskins TW, Davies JR, Allchin A, Miller CL, Pollock TM. Controlled trial of inactivated influenza vaccine containing the a‐Hong Kong strain during an outbreak of influenza due to the a‐England‐42‐72 strain. Lancet 1973;2(7821):116‐20. - PubMed
Hoskins 1979 {published data only}
-
- Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza‐A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet 1979;1(8106):33‐5. - PubMed
Howell 1964a {published data only}
Howell 1964b {published data only}
Hrabar 1977 {published data only}
-
- Hrabar A, Vodopija I, Andre FE, Mitchell JR, Maassab HF, Hennessy AV, et al. A placebo‐controlled dose‐response study of the reactogenicity and immunogenicity of a cold‐adapted recombinant A/Victoria/3/75 (H3N2) live influenza virus candidate vaccine in healthy volunteers. Developments in Biological Standardization 1977;39:53‐60. - PubMed
Hurwitz 2000a {published data only}
-
- Hurwitz ES, Haber M, Chang A, Shope T, Teo S, Ginsberg M, et al. Effectiveness of influenza vaccination of day care children in reducing influenza‐related morbidity among household contacts. JAMA 2000;284(13):1677‐82. - PubMed
Hurwitz 2000b {published data only}
-
- Hurwitz ES, Haber M, Chang A, Shope T, Teo ST, Giesick JS, et al. Studies of the 1996‐1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. Journal of Infectious Diseases 2000;182:1218‐21. - PubMed
Jansen 2008 {published data only}
-
- Jansen AG, Sanders EA, Hoes AW, Loon AM, Hak E. Effects of influenza plus pneumococcal conjugate vaccination versus influenza vaccination alone in preventing respiratory tract infections in children: a randomized, double‐blind, placebo‐controlled trial. Journal of Pediatrics 2008;153(6):764‐70. - PMC - PubMed
Jovanovic 1979 {published data only}
-
- Jovanovic D, Georges‐Delvaux AM, Delem A. Protective efficacy of live influenza vaccines. Its assessment in field and experimental conditions. Developments in Biological Standardization 1979;43:241‐5. - PubMed
Jurgenssen 1978 {published data only}
-
- Jurgenssen O, Moritz A, Liehl E, Bachmayer H. Vaccination of infants and schoolchildren with an influenza subunit vaccine (author's translation). Infection 1978;6(5):221‐7. - PubMed
Just 1978 {published data only}
-
- Just M, Burgin‐Wolff A, Berger‐Hernandez R, Bachlin A, Ritzel G, Moritz AJ. A/New Jersey/76 influenza vaccine trial in seronegative schoolchildren: comparison of a subunit vaccine with a whole‐virus vaccine. Medical Microbiology and Immunology 1978;164(4):277‐84. - PubMed
Karron 1995 {published data only}
-
- Karron RA, Steinhoff MC, Subbarao EK, Wilson MH, Macleod K, Clements ML, et al. Safety and immunogenicity of a cold‐adapted influenza A (H1N1) reassortant virus vaccine administered to infants less than six months of age. Pediatric Infectious Disease Journal 1995;14(1):10‐6. - PubMed
Kaufman 2000 {published data only}
-
- Kaufman Z, Cohen‐Manheim I, Green MS. Compliance with influenza vaccination in Israel in two successive winters, 1998/1999 and 1999/2000. Israel Medical Association Journal 2000;2(10):742‐5. - PubMed
King 2001 {published data only}
-
- King JC Jr, Fast PE, Zangwill KM, Weinberg GA, Wolff M, Yan L, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold‐adapted, live attenuated influenza vaccine administered to human immunodeficiency virus‐infected and noninfected children. Pediatric Infectious Disease Journal 2001;20(12):1124‐31. - PubMed
Kissling 2011a {published data only}
-
- Kissling E, Valenciano M. Early estimates of seasonal influenza vaccine effectiveness in Europe, 2010/11: I‐MOVE, a multicentre case‐control study. Eurosurveillance 2011;16:11. - PubMed
Kramarz 2001 {published data only}
-
- Kramarz P, Destefano F, Gargiullo PM, Chen RT, Lieu TA, Davis RL, et al. Does influenza vaccination prevent asthma exacerbations in children?. Journal of Pediatrics 2001;138(3):306‐10. - PubMed
Kuno‐Sakai 1994 {published data only}
-
- Kuno‐Sakai H, Kimura M, Ohta K, Shimojima R, Oh Y, Fukumi H. Developments in mucosal influenza virus vaccines. Vaccine 1994;12(14):1303‐10. - PubMed
La Montagne 1983 {published data only}
-
- Montagne JR, Noble GR, Quinnan GV, Curlin GT, Blackwelder WC, Smith JI, et al. Summary of clinical trials of inactivated influenza vaccine ‐ 1978. Reviews of Infectious Diseases 1983;5(4):723‐36. - PubMed
Lauteria 1974 {published data only}
-
- Lauteria SF, Kantzler GB, High PC, Lee JD, Waldman RH. An attenuated influenza virus vaccine: reactogenicity, transmissibility, immunogenicity, and protective efficacy. Journal of Infectious Diseases 1974;130(4):380‐3. - PubMed
Lerman 1977 {published data only}
-
- Lerman SJ. Reactivity and immunogenicity of monovalent A/New Jersey/76 influenza virus vaccines in children. Journal of Infectious Diseases 1977;136(Suppl):563‐70. - PubMed
Lina 2000 {published data only}
-
- Lina B, Fletcher MA, Valette M, Saliou P, Aymard M. A TritonX‐100‐split virion influenza vaccine is safe and fulfills the Committee for Proprietary Medicinal Products (CPMP) recommendations for the European Community for immunogenicity, in children, adults and the elderly. Biologicals 2000;28(2):95‐103. - PubMed
Longini 2000 {published data only}
-
- Longini IM, Halloran ME, Nizam A, Wolff M, Mendelman PM, Fast PE, et al. Estimation of the efficacy of live, attenuated influenza vaccine from a two‐year, multi‐center vaccine trial: implications for influenza epidemic control. Vaccine 2000;18(18):1902‐9. - PubMed
Luce 2001 {published data only}
-
- Luce BR, Zangwill KM, Palmer CS, Mendelman PM, Yan L, Wolff MC, et al. Cost‐effectiveness analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children. Pediatrics 2001;108(2):e24. - PubMed
Luthardt 1979 {published data only}
-
- Luthardt T, Lutz O, Kindt H. Immunisation against influenza with a new subunit vaccine tested on children at risk (author's translation). Deutsche Medizinische Wochenschrift 1979;10(2):56‐60. - PubMed
Madhi 2014 {published data only}
-
- Madhi S, Cutland C, Jones S, Hugo A, Treurnicht FK, Kuwanda L, et al. Randomized, placebo‐controlled trial on safety and efficacy of inactivated influenza vaccination of pregnant women in preventing illness in their infants. International Journal of Infectious Diseases 2014;21:32.
Marchisio 2002 {published data only}
-
- Marchisio P, Cavagna R, Maspes B, Gironi S, Esposito S, Lambertini L, et al. Efficacy of intranasal virosomal influenza vaccine in the prevention of recurrent acute otitis media in children. Clinical Infectious Diseases 2002;35(2):168‐74. - PubMed
Martin Moreno 1998 {published data only}
-
- Martín Moreno V, Molina Cabrerizo MR, Sotillo Rincón MJ, Gómez Gómez C, Alvarez Gómez J. Adverse effects associated with influenza vaccine in pediatrics. Revista Española de Salud Pública 1998;72:319‐29. - PubMed
Maynard 1968 {published data only}
-
- Maynard JE, Dull HB, Hanson ML, Feltz ET, Berger R, Hammes L. Evaluation of monovalent and polyvalent influenza vaccines during an epidemic of type A2 and B influenza. American Journal of Epidemiology 1968;87(1):148‐57. - PubMed
McMahon 2008 {published data only}
-
- McMahon AW, Iskander JK, Haber P, Braun MM, Ball R. Inactivated influenza vaccine (IIV) in children < 2 years of age: examination of selected adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) after thimerosal‐free or thimerosal‐containing vaccine. Vaccine 2008;26(3):427‐9. - PubMed
Mendelman 2001 {published data only}
-
- Mendelman PM, Cordova J, Cho I. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold‐adapted (CAIV‐T) in healthy children and healthy adults. Vaccine 2001;19(17‐9):2221‐6. - PubMed
Monto 1970 {published data only}
-
- Monto AS, Davenport FM, Napier JA, Francis T Jr. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. Journal of Infectious Diseases 1970;122(1):16‐25. - PubMed
Monto 1977 {published data only}
-
- Monto AS, Maassab HF. Use of influenza vaccine in non‐high risk populations. Developments in Biological Standardization 1977;39:329‐35. - PubMed
Morio 1994 {published data only}
Morris 1976 {published data only}
-
- Morris CA, Freestone DS, Stealey VM. Natural challenge of subjects vaccinated with WRL 105 strain live influenza vaccine in a residential community. Developments in Biological Standardization 1976;33:197‐201. - PubMed
Muhammad 2011 {published data only}
-
- Muhammad RD, Haber P, Broder KR, Leroy Z, Ball R, Braun MM, et al. Adverse events following trivalent inactivated influenza vaccination in children: analysis of the Vaccine Adverse Event Reporting System. Pediatric Infectious Disease Journal 2011;30(1):e1‐8. - PubMed
Neuzil 2001 {published data only}
-
- Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold‐adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatric Infectious Disease Journal 2001;20(8):733‐40. - PubMed
Neuzil 2006 {published data only}
-
- Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine‐naive 5‐8‐year‐old children. Journal of Infectious Diseases 2006;194:1032‐9. - PubMed
Nolan 2003 {published data only}
-
- Nolan T, Lee MS, Cordova JM, Cho I, Walker RE, August MJ, et al. Safety and immunogenicity of a live‐attenuated influenza vaccine blended and filled at two manufacturing facilities. Vaccine 2003;21(11‐2):1224‐31. - PubMed
Ogra 1977 {published data only}
-
- Ogra PL, Chow T, Beutner KR, Rubi E, Strussenberg J, DeMello S, et al. Clinical and immunologic evaluation of neuraminidase‐specific influenza A virus vaccine in humans. Journal of Infectious Diseases 1977;135(4):499‐506. - PubMed
Piedra 1991 {published data only}
-
- Piedra PA, Glezen PW. Influenza in children: epidemiology, immunity, and vaccines. Seminars in Pediatric Infectious Diseases 1991;2(2):140‐6.
Piedra 1993 {published data only}
-
- Piedra PA, Glezen WP, Mbawuike I, Gruber WC, Baxter BD, Boland FJ, et al. Studies on reactogenicity and immunogenicity of attenuated bivalent cold recombinant influenza type A (CRA) and inactivated trivalent influenza virus (TI) vaccines in infants and young children. Vaccine 1993;11(7):718‐24. - PubMed
Piedra 2002b {published data only}
-
- Piedra PA. Safety of the trivalent, cold‐adapted influenza vaccine (CAIV‐T) in children. Seminars in Pediatric Infectious Diseases 2002;13(2):90‐6. - PubMed
Quach 2003 {published data only}
-
- Quach C, Piche‐Walker L, Platt R, Moore D. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics 2003;112(3 Pt 1):e197‐201. - PubMed
Rimmelzwaan 2000 {published data only}
-
- Rimmelzwaan GF, Nieuwkoop N, Brandenburg A, Sutter G, Beyer WE, Maher D, et al. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine 2000;19(9‐10):1180‐7. - PubMed
Ruben 1973 {published data only}
-
- Ruben FL, Akers LW, Stanley ED, Jackson GG. Protection with split and whole virus vaccines against influenza. Archives of Internal Medicine 1973;132(4):568‐71. - PubMed
Schaad 2000 {published data only}
-
- Schaad UB, Buhlmann U, Burger R, Ruedeberg A, Wilder‐Smith A, Rutishauser M, et al. Comparison of immunogenicity and safety of a virosome influenza vaccine with those of a subunit influenza vaccine in pediatric patients with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2000;44(5):1163‐7. - PMC - PubMed
Scheifele 1990 {published data only}
Schiff 1975 {published data only}
Slepushkin 1993 {published data only}
-
- Slepushkin AN, Obrosova‐Serova NP, Burtseva EI, Rudenko LG, Govorkova EA, Vartanyan RV, et al. Comparison of live attenuated and inactivated influenza vaccines in schoolchildren in Russia: I. Safety and efficacy in two Moscow schools, 1987/88. Vaccine 1993;11(3):323‐8. - PubMed
Stowe 2011 {published data only}
-
- Stowe J, Andrews N, Bryan P, Seabroke S, Miller E. Risk of convulsions in children after monovalent H1N1 (2009) and trivalent influenza vaccines: a database study. Vaccine 2011;29(51):9467‐72. - PubMed
Sugaya 1994 {published data only}
-
- Sugaya N, Nerome K, Ishida M, Matsumoto M, Mitamura K, Nirasawa M. Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well‐matched type B. JAMA 1994;272(14):1122‐6. - PubMed
Sumaya 1977 {published data only}
-
- Sumaya CV, Brunell PA. A clinical trial with monovalent influenza A/New Jersey/76 virus vaccine in preschool and school‐age children. Journal of Infectious Diseases 1977;Suppl 136:597‐600. - PubMed
Van Hoecke 1996 {published data only}
-
- Hoecke C, Raue W, Kunzel W, Engelmann H. Immunogenicity and safety of influenza vaccination in 3‐ to 6‐year‐old children with a two dose immunisation schedule. European Journal of Pediatrics 1996;155(4):346‐7. - PubMed
Vasil'eva 1986 {published data only}
-
- Vasil'eva RI, Liamtseva GA, Riazantseva TG, Oleinikova EV, Sosunov AV. Assessment of the prophylactic effectiveness of an inactivated influenza vaccine by immunizing schoolchildren in the springtime. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1986;3:10‐4. - PubMed
Vasil'eva 1987 {published data only}
-
- Vasil'eva RI, Lonskaia NI, Nefedova LA, Iaroshevskaia II, Bichurina MA. Results of a study of new preparations of inactivated whole‐virion influenza vaccines for the protection of children in the USSR. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1987;3:38‐42. - PubMed
Wahlberg 2003 {published data only}
-
- Wahlberg J, Fredriksson J, Vaarala O, Ludvigsson J. Vaccinations may induce diabetes‐related autoantibodies in one‐year‐old children. Annals of the New York Academy of Sciences 2003;1005:404‐8. - PubMed
Welty 1977a {published data only}
-
- Welty PB Jr, Epstein B, O'Brien J, Brackett RG, Brandon FB, Shillis JL. Reactions and serologic responses after administration of inactivated monovalent influenza A/swine virus vaccines. II. Immunization of children with influenza A/New Jersey/X‐53 virus vaccines. Journal of Infectious Diseases 1977;136(Suppl):609‐11. - PubMed
Welty 1977b {published data only}
-
- Welty PB Jr, Epstein B, O'Brien J, Brackett RG, Brandon FB, Shillis JL. Reactions and serologic responses after administration of inactivated monovalent influenza A/swine virus vaccines. I. Immunization of children and adults with influenza A/Shope virus vaccines. Journal of Infectious Diseases 1977;136(Suppl):604‐8. - PubMed
Wesselius‐de 1972 {published data only}
Wright 1976b {published data only}
-
- Wright PF, Dolin R, Montagne JR. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines‐II. Journal of Infectious Diseases 1976;134:633‐8. - PubMed
Wright 1985 {published data only}
-
- Wright PF, Bhargava M, Johnson PR, Thompson J, Karzon DT. Simultaneous administration of live, attenuated influenza A vaccines representing different serotypes. Vaccine 1985;3(3):305‐8. - PubMed
Wu 2010 {published data only}
-
- Wu J, Xu F, Lu L, Lu M, Miao L, Gao T, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. New England Journal of Medicine 2010;363(25):2416‐23. - PubMed
Zhilova 1986 {published data only}
-
- Zhilova GP, Ignat'eva GS, Orlov VA, Malikova EV, Maksakova VL. Results of a study of the effectiveness of simultaneous immunization against influenza with live and inactivated vaccines (1980‐1983). Voprosy Virusologii 1986;31(1):40‐4. - PubMed
Additional references
AAPCID 2004
-
- American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for influenza immunization of children. Pediatrics 2004;113:1441‐7. - PubMed
ACIP 2016
-
- Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines. Morbidity and Mortality Weekly Report 2016;65(5):1‐54. - PubMed
Ambrose 2012
-
- Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta‐analysis of 8 randomized controlled studies. Vaccine 2012;30(5):886‐92. - PubMed
Atkins 2004
Belshe 2010
Broome 1980
-
- Broome C, Facklam R, Fraser D. Pneumococcal disease after pneumococcal vaccination ‐ an alternative method to estimate the efficacy of pneumococcal vaccine. New England Journal of Medicine 1980;303(10):549‐52. - PubMed
Carter 2011
-
- Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist; Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011;71(12):1591‐622. - PubMed
CDC 2007
-
- Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. Morbidity and Mortality Weekly Report 2007;56(RR‐6):1‐54. - PubMed
CDC 2011
-
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. Morbidity and Mortality Weekly Report 2011;60(33):1128‐32. - PubMed
Collignon 2010
-
- Collignon P, Doshi P, Jefferson T. Ramifications of adverse events in children in Australia (letter to the Editor). BMJ 2010;340:c2994. - PubMed
Cowling 2012
Demicheli 2010
Demicheli 2014
ECDC 2009
-
- European Centre for Disease Prevention and Control. Protocol for case‐control studies to measure pandemic and seasonal influenza vaccine effectiveness in the European Union and European Economic Area Member States ‐ Technical Document. ecdc.europa.eu/en/publications/Publications/0907_TED_Influenza_AH1N1_Mea... (accessed prior to 23 January 2018).
EMA 2014
-
- European Medicines Agency. EMA/CHMP/VWP/457259/2014. Committee for Medicinal Products for Human Use. Guideline on influenza vaccines. Non‐clinical and clinical module. www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webConte... (accessed prior to 23 January 2018).
Fiore 2011
-
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. Morbidity and Mortality Weekly Report 2010;59(RR‐8):1‐62. - PubMed
Foppa 2013
-
- Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test‐negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013;31(30):3104‐9. - PubMed
Forrest 2008
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Harper 2004
-
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB, Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2004;53(RR‐6):1‐40. - PubMed
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ioannidis 2011
Izurieta 2000
-
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. New England Journal of Medicine 2000;342:232‐9. - PubMed
Jefferson 2005a
-
- Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A. Safety of influenza vaccines in children. Lancet 2005;366(9488):803‐4. - PubMed
Jefferson 2009
Jefferson 2010
Jefferson 2012a
Jordan 2006
-
- Jordan R, Connock M, Albon E, Fry‐Smith A, Olowokure B, Hawker J, et al. Universal vaccination of children against influenza: are there indirect benefits to the community? A systematic review of the evidence. Vaccine 2006;24(8):1047‐62. - PubMed
Kissling 2017
-
- Kissling E, Rondy M, I‐MOVE/I‐MOVE+ study team. Early 2016/17 vaccine effectiveness estimates against influenza A(H3N2): I‐MOVE multicentre case control studies at primary care and hospital levels in Europe. Eurosurveillance 2017;22(7):30464. [DOI: 10.2807/1560-7917.ES.2017.22.7.30464] - DOI - PMC - PubMed
Loeb 2010
-
- Loeb M, Russell ML, Moss L, Fonseca K, Fox J, Earn DJD, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010;303(10):943–50. - PubMed
Lum 2010
-
- Lum LC, Borja‐Tabora CF, Breiman RF, Vesikari T, Sablan BP, Chay OM, et al. Influenza vaccine concurrently administered with a combination measles, mumps, and rubella vaccine to young children. Vaccine 2010;28(6):1566‐74. - PubMed
Manzoli 2007
-
- Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: a meta‐analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatric Infectious Disease Journal 2007;26(2):97‐106. - PubMed
Manzoli 2011
Mereckiene 2010
-
- Mereckiene J, Cotter S, D’Ancona F, Giambi C, Nicoll A, Levy‐Bruhl D, et al. Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008‐2009. Eurosurveillance 2010;15(44):1‐10. - PubMed
Michiels 2011
-
- Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 2011;29(49):9159‐70. - PubMed
Negri 2005
-
- Negri E, Colombo C, Giordano L, Groth N, Apolone G, Vecchia C. Influenza vaccine in healthy children: a meta‐analysis. Vaccine 2005;23(22):2851‐61. - PubMed
Neuzil 2000
-
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. New England Journal of Medicine 2000;342:225‐31. - PubMed
Orenstein 1985
Orr 2004
-
- Orr P. Statement on influenza vaccination for the 2004‐2005 season. Canada Communicable Disease Report 2004;30:1‐32. - PubMed
Osterholm 2012
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta‐analysis. Lancet 2012;12(1):36‐44. - PubMed
Perencevich 2013a
-
- Perencevich EN. Pondering vexing issues in infection prevention and control. haicontroversies.blogspot.com.au/2015/09/there‐is‐no‐such‐thing‐as‐patie... (accessed 22 May 2017).
Perencevich 2013b
-
- Perencevich EN. How should we calculate influenza vaccine effectiveness? ACP Internist Blog Archive. blog.acpinternist.org/2013/02/how‐should‐we‐calculate‐influenza_7.html (accessed 17 May 2017).
Principi 2004
-
- Principi N, Esposito S. Are we ready for universal influenza vaccination in paediatrics?. Lancet 2004;4:75‐83. - PubMed
Reichert 2001
-
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. New England Journal of Medicine 2001;344:889‐96. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhorer 2009
-
- Rhorer J, Ambrose CS, Dickinson S, Hamilton H, Oleka NA, Malinoski FJ, et al. Efficacy of live attenuated influenza vaccine in children: a meta‐analysis of nine randomized clinical trials. Vaccine 2009;27(7):1101‐10. - PubMed
Rodrigo Pendas 2007
-
- Rodrigo Pendas JA, Alemany Vilches L, Moraga Llop FA. Systematic reviews of the efficacy and effectiveness of influenza vaccination in infants, children and teenagers [Revisiones sistematicas de la eficacia o efectividad de las vacunas antigripales en el lactante, el nino y el adolescente sano]. Vacunas 2007;8(3):130‐42.
THL 2012
-
- National Institute for Health and Welfare (THL). National Narcolepsy Task Force Interim Report. www.thl.fi/thl‐client/pdfs/dce182fb‐651e‐48a1‐b018‐3f774d6d1875 (accessed 2 January 2012).
Treanor 2016
Valenciano 2012
-
- Valenciano M, Ciancio BC, I‐MOVE study team. I‐MOVE: a European network to measure the effectiveness of influenza vaccine. Eurosurveillance 2012;17(39):20281. - PubMed
Wijnans 2011
-
- Wijnans L, Bie S, Dieleman J, Bonhoeffer J, Sturkenboom M. Safety of pandemic H1N1 vaccines in children and adolescents. Vaccine 2011;29(43):7559‐71. - PubMed
Wijnans 2016
Wiselka 1994
References to other published versions of this review
Jefferson 2005b
-
- Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet 2005;365(9461):773‐80. - PubMed
Jefferson 2008
Jefferson 2012b
Smith 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

