Vaccines for preventing influenza in healthy adults
- PMID: 29388196
- PMCID: PMC6491184
- DOI: 10.1002/14651858.CD001269.pub6
Vaccines for preventing influenza in healthy adults
Abstract
Background: The consequences of influenza in adults are mainly time off work. Vaccination of pregnant women is recommended internationally. This is an update of a review published in 2014. Future updates of this review will be made only when new trials or vaccines become available. Observational data included in previous versions of the review have been retained for historical reasons but have not been updated due to their lack of influence on the review conclusions.
Objectives: To assess the effects (efficacy, effectiveness, and harm) of vaccines against influenza in healthy adults, including pregnant women.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12), MEDLINE (January 1966 to 31 December 2016), Embase (1990 to 31 December 2016), the WHO International Clinical Trials Registry Platform (ICTRP; 1 July 2017), and ClinicalTrials.gov (1 July 2017), as well as checking the bibliographies of retrieved articles.
Selection criteria: Randomised controlled trials (RCTs) or quasi-RCTs comparing influenza vaccines with placebo or no intervention in naturally occurring influenza in healthy individuals aged 16 to 65 years. Previous versions of this review included observational comparative studies assessing serious and rare harms cohort and case-control studies. Due to the uncertain quality of observational (i.e. non-randomised) studies and their lack of influence on the review conclusions, we decided to update only randomised evidence. The searches for observational comparative studies are no longer updated.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We rated certainty of evidence for key outcomes (influenza, influenza-like illness (ILI), hospitalisation, and adverse effects) using GRADE.
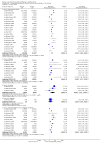
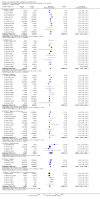
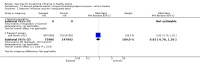
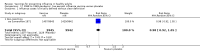
Main results: We included 52 clinical trials of over 80,000 people assessing the safety and effectiveness of influenza vaccines. We have presented findings from 25 studies comparing inactivated parenteral influenza vaccine against placebo or do-nothing control groups as the most relevant to decision-making. The studies were conducted over single influenza seasons in North America, South America, and Europe between 1969 and 2009. We did not consider studies at high risk of bias to influence the results of our outcomes except for hospitalisation.Inactivated influenza vaccines probably reduce influenza in healthy adults from 2.3% without vaccination to 0.9% (risk ratio (RR) 0.41, 95% confidence interval (CI) 0.36 to 0.47; 71,221 participants; moderate-certainty evidence), and they probably reduce ILI from 21.5% to 18.1% (RR 0.84, 95% CI 0.75 to 0.95; 25,795 participants; moderate-certainty evidence; 71 healthy adults need to be vaccinated to prevent one of them experiencing influenza, and 29 healthy adults need to be vaccinated to prevent one of them experiencing an ILI). The difference between the two number needed to vaccinate (NNV) values depends on the different incidence of ILI and confirmed influenza among the study populations. Vaccination may lead to a small reduction in the risk of hospitalisation in healthy adults, from 14.7% to 14.1%, but the CI is wide and does not rule out a large benefit (RR 0.96, 95% CI 0.85 to 1.08; 11,924 participants; low-certainty evidence). Vaccines may lead to little or no small reduction in days off work (-0.04 days, 95% CI -0.14 days to 0.06; low-certainty evidence). Inactivated vaccines cause an increase in fever from 1.5% to 2.3%.We identified one RCT and one controlled clinical trial assessing the effects of vaccination in pregnant women. The efficacy of inactivated vaccine containing pH1N1 against influenza was 50% (95% CI 14% to 71%) in mothers (NNV 55), and 49% (95% CI 12% to 70%) in infants up to 24 weeks (NNV 56). No data were available on efficacy against seasonal influenza during pregnancy. Evidence from observational studies showed effectiveness of influenza vaccines against ILI in pregnant women to be 24% (95% CI 11% to 36%, NNV 94), and against influenza in newborns from vaccinated women to be 41% (95% CI 6% to 63%, NNV 27).Live aerosol vaccines have an overall effectiveness corresponding to an NNV of 46. The performance of one- or two-dose whole-virion 1968 to 1969 pandemic vaccines was higher (NNV 16) against ILI and (NNV 35) against influenza. There was limited impact on hospitalisations in the 1968 to 1969 pandemic (NNV 94). The administration of both seasonal and 2009 pandemic vaccines during pregnancy had no significant effect on abortion or neonatal death, but this was based on observational data sets.
Authors' conclusions: Healthy adults who receive inactivated parenteral influenza vaccine rather than no vaccine probably experience less influenza, from just over 2% to just under 1% (moderate-certainty evidence). They also probably experience less ILI following vaccination, but the degree of benefit when expressed in absolute terms varied across different settings. Variation in protection against ILI may be due in part to inconsistent symptom classification. Certainty of evidence for the small reductions in hospitalisations and time off work is low. Protection against influenza and ILI in mothers and newborns was smaller than the effects seen in other populations considered in this review.Vaccines increase the risk of a number of adverse events, including a small increase in fever, but rates of nausea and vomiting are uncertain. The protective effect of vaccination in pregnant women and newborns is also very modest. We did not find any evidence of an association between influenza vaccination and serious adverse events in the comparative studies considered in this review. Fifteen included RCTs were industry funded (29%).
Conflict of interest statement
Vittorio Demicheli: none known
Tom Jefferson (TJ) was a co‐recipient of a UK National Institute for Health Research grant (HTA – 10/80/01 Update and amalgamation of two Cochrane Reviews: neuraminidase inhibitors for preventing and treating influenza in healthy adults and children (
Eliana Ferroni: none known
Alessandro Rivetti: none known
Carlo Di Pietrantonj: none known
Figures






















































Update of
-
Vaccines for preventing influenza in healthy adults.Cochrane Database Syst Rev. 2014 Mar 13;(3):CD001269. doi: 10.1002/14651858.CD001269.pub5. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Feb 01;2:CD001269. doi: 10.1002/14651858.CD001269.pub6. PMID: 24623315 Updated.
References
References to studies included in this review
aa Barrett 2011 {published data only}
-
- Barrett PN, Berezuk G, Fritsch S, Aichinger G, Hart MK, El‐Amin W, et al. Efficacy, safety, and immunogenicity of a Vero‐cell‐culture‐derived trivalent influenza vaccine: a multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet 2011;377(9767):751‐9. - PubMed
-
- Ehrlich HJ, Berezuk G, Fritsch S, Aichinger G, Singer J, Portsmouth D, et al. Clinical development of a Vero cell culture‐derived seasonal influenza vaccine. Vaccine 2011;30(29):4377‐86. - PubMed
aa Beran 2009a {published data only}
-
- Beran J, Wertzova V, Honegr K, Kaliskova E, Havlickova M, Havlik J, et al. Challenge of conducting a placebo‐controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infectious Diseases 2009;9:2. [DOI: 10.1186/1471-2334-9-2] - DOI - PMC - PubMed
aa Beran 2009b {published data only}
-
- Beran J, Vesikari T, Wertzova V, Karvonen A, Honegr K, Lindblad N, et al. Efficacy of inactivated split‐virus influenza vaccine against culture‐confirmed influenza in healthy adults: a prospective, randomized, placebo‐controlled trial. Journal of Infectious Diseases 2009;200:1861‐9. - PubMed
aa Bridges 2000a {published data only}
-
- Buxton Bridges C, Thompson VV, Meltzer MI, Reeve GR, Talamonti VJ, Cox NJ, et al. Effectiveness and cost benefit of influenza vaccination of healthy working adults, a randomized controlled trial. JAMA 2000;284(13):1655‐63. - PubMed
aa Bridges 2000b {published data only}
-
- Buxton Bridges C, Thompson VV, Meltzer MI, Reeve GR, Talamonti VJ, Cox NJ. Effectiveness and cost benefit of influenza vaccination of healthy working adults, a randomized controlled trial. JAMA 2000;284(13):1655‐63. - PubMed
aa Eddy 1970 {published data only}
-
- Eddy TS, Davies NA. The effect of vaccine on a closed epidemic of Hong Kong influenza. South African Medical Journal 1970;44(8):214‐6. - PubMed
aa Edwards 1994a {published data only}
-
- Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold adapted and inactivated vaccines for the prevention of influenza A disease. Journal of Infectious Diseases 1994;169(1):68‐76. - PubMed
aa Edwards 1994b {published data only}
-
- Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold adapted and inactivated vaccines for the prevention of influenza A disease. Journal of Infectious Diseases 1994;169(1):68‐76. - PubMed
aa Edwards 1994c {published data only}
-
- Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold adapted and inactivated vaccines for the prevention of influenza A disease. Journal of Infectious Diseases 1994;169(1):68‐76. - PubMed
aa Edwards 1994d {published data only}
-
- Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold adapted and inactivated vaccines for the prevention of influenza A disease. Journal of Infectious Diseases 1994;169(1):68‐76. - PubMed
aa Frey 2010 {published data only}
-
- Frey S, Vesikari T, Szymczakiewicz‐Multanowska A, Lattanzi M, Izu A, Groth N, et al. Clinical efficacy of cell culture‐derived and egg‐derived inactivated subunit influenza vaccines in healthy adults. Clinical Infectious Diseases 2010;51(9):997‐1004. - PubMed
aa Hammond 1978 {published data only}
-
- Hammond ML, Ferris AA, Faine S. Effective protection against influenza after vaccination with subunit vaccine. Medical Journal of Australia 1978;1(6):301‐3. - PubMed
aa Jackson 2010a {published data only}
aa Jackson 2010b {published data only}
aa Keitel 1988a {published data only}
-
- Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. American Journal of Epidemiology 1988;127(2):353‐64. - PubMed
aa Keitel 1988b {published data only}
-
- Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. American Journal of Epidemiology 1988;127(2):353‐64. - PubMed
aa Keitel 1997a {published data only}
-
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997;15(10):1114‐22. - PubMed
aa Keitel 1997b {published data only}
-
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997;15(10):1114‐22. - PubMed
aa Keitel 1997c {published data only}
-
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997;15(10):1114‐22. - PubMed
aa Langley 2011 {published data only}
-
- Langley JM, Aoki F, Ward BJ, McGeer A, Angel JB, Stiver G, et al. A nasally administered trivalent inactivated influenza vaccine is well tolerated, stimulates both mucosal and systemic immunity, and potentially protects against influenza illness. Vaccine 2011;29(10):1921‐8. - PubMed
aa Leibovitz 1971 {published data only}
-
- Leibovitz A, Coultrip RL, Kilbourne ED, Legters LJ, Smith CD, Chin J, et al. Correlated studies of a recombinant influenza‐virus vaccine. IV. Protection against naturally occurring influenza in military trainees. Journal of Infectious Diseases 1971;124(5):481‐7. - PubMed
aa Mcbride 2016a {published data only}
-
- Mcbride WJ, Abhayaratna WP, Barr I, Booy R, Carapetis J, Carson S, et al. Efficacy of a trivalent influenza vaccine against seasonal strains and against 2009 pandemic H1N1: a randomized, placebo‐controlled trial. Vaccine 2016;34(41):4991‐7. - PubMed
aa Mcbride 2016b {published data only}
-
- Mcbride WJ, Abhayaratna WP, Barr I, Booy R, Carapetis J, Carson S, et al. Efficacy of a trivalent influenza vaccine against seasonal strains and against 2009 pandemic H1N1: a randomized, placebo‐controlled trial. Vaccine 2016;34(41):4991‐7. - PubMed
aa Mesa Duque 2001 {published data only}
-
- Mesa Duque SS, Moreno AP, Hurtado G, Arbelàaz Montoya MP. Effectiveness of an influenza vaccine in a working population in Colombia [Effectividad de una vacuna anti gripal en una poblaciòn laboral colombiana]. Pan American Journal of Public Health 2001;10(4):232‐9. - PubMed
aa Mixéu 2002 {published data only}
-
- Mixéu MA, Vespa GN, Forleo‐Neto E, Toniolo‐Neto J, Alves PM. Impact of influenza vaccination on civilian aircrew illness and absenteeism. Aviation, Space, and Environmental Medicine 2002;73(9):876‐80. - PubMed
aa Mogabgab 1970a {published data only}
-
- Mogabgab WJ, Leiderman E. Immunogenicity of 1967 polyvalent and 1968 Hong Kong influenza vaccines. JAMA 1970;211(10):1672‐6. - PubMed
aa Mogabgab 1970b {published data only}
-
- Mogabgab WJ, Leiderman E. Immunogenicity of 1967 polyvalent and 1968 Hong Kong influenza vaccines. JAMA 1970;211(10):1672‐6. - PubMed
aa Monto 1982 {published data only}
-
- Monto AS, DeWolfe Miller F, Maassab HF. Evaluation of an attenuated, cold recombinant influenza B virus vaccine. Journal of Infectious Diseases 1982;145(1):57‐64. - PubMed
aa Monto 2009 {published data only}
-
- Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. New England Journal of Medicine 2009;361(13):1260‐7. - PubMed
aa Nichol 1995 {published data only}
-
- Nichol KL, Lind A, Margolis KL, Murdoch M, McFadden R, Hauge M. The effectiveness of vaccination against influenza in healthy, working adults. New England Journal of Medicine 1995;333(14):889‐93. - PubMed
aa Nichol 1999a {published data only}
-
- Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, et al. Effectiveness of live attenuated intranasal influenza virus vaccine in healthy working adults, a randomized controlled trial. JAMA 1999;282(2):137‐44. - PubMed
aa Ohmit 2006 {published data only (unpublished sought but not used)}
aa Ohmit 2008 {published data only}
aa Powers 1995a {published data only}
-
- Powers DC, Smith GE, Anderson EL, Kennedy DJ, Hackett CS, Wilkinson BE, et al. Influenza A virus vaccines containing purified recombinant H3 hemagglutinin are well tolerated and induce protective immune responses in healthy adults. Journal of Infectious Diseases 1995;171(6):1595‐9. - PubMed
aa Powers 1995b {published data only}
-
- Powers DC, Smith GE, Anderson EL, Kennedy DJ, Hackett CS, Wilkinson BE, et al. Influenza A virus vaccines containing purified recombinant H3 hemagglutinin are well tolerated and induce protective immune responses in healthy adults. Journal of Infectious Diseases 1995;171(6):1595‐9. - PubMed
aa Powers 1995c {published data only}
-
- Powers DC, Smith GE, Anderson EL, Kennedy DJ, Hackett CS, Wilkinson BE, et al. Influenza A virus vaccines containing purified recombinant H3 hemagglutinin are well tolerated and induce protective immune responses in healthy adults. Journal of Infectious Diseases 1995;171:1595‐9. - PubMed
aa Rytel 1977 {published data only}
-
- Rytel MW, Jackson LJ, Niebojewski RA, Haagensen JL, Rosenkranz MA. Field trial of live attenuated influenza A/B ("Alice"/R‐75) vaccine. American Journal of Epidemiology 1977;105(1):49‐55. - PubMed
aa Sumarokow 1971 {published data only}
-
- Sumarokow AA, Popov VF, Nefedova LA, Salmin LV, Lazorenko NF. A study of live influenza vaccines in a controlled trial. Zhumal Mikrobiologii Epidemiologii Immunobiologii 1971;48(2):46‐52. - PubMed
aa Tannock 1984 {published data only}
-
- Tannock GA, Bryce DA, Hensley MJ, Saunders NA, Gillett RS, Kennedy WS. Responses to one or two doses of a deoxycholate subunit influenza vaccine in a primed population. Vaccine 1984;2(1):100‐5. - PubMed
aa Treanor 2011 {published data only}
-
- Treanor JJ, Sahly H, King J, Graham I, Izikson R, Kohberger R, et al. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok) against influenza in healthy adults: a randomised, placebo‐controlled trial. Vaccine 2011;29(44):7733‐9. - PubMed
aa Waldman 1969a {published data only}
aa Waldman 1969b {published data only}
aa Waldman 1969c {published data only}
aa Waldman 1969d {published data only}
aa Waldman 1972a {published data only}
-
- Waldman RH, Coggins WJ. Influenza immunization: field trial on a university campus. Journal of Infectious Diseases 1972;126(3):242‐8. - PubMed
aa Waldman 1972b {published data only}
-
- Waldman RH, Coggins WJ. Influenza immunization: field trial on a university campus. Journal of Infectious Diseases 1972;126(3):242‐8. - PubMed
aa Waldman 1972c {published data only}
-
- Waldman RH, Coggins WJ. Influenza immunization: field trial on a university campus. Journal of Infectious Diseases 1972;126(3):242‐8. - PubMed
aa Waldman 1972d {published data only}
-
- Waldman RH, Coggins WJ. Influenza immunization: field trial on a university campus. Journal of Infectious Diseases 1972;126(3):242‐8. - PubMed
aa Weingarten 1988 {published data only}
-
- Weingarten S, Staniloff H, Ault M, Miles P, Bamberger M, Meyer RD. Do hospital employees benefit from the influenza vaccine?. Journal of General Internal Medicine 1988;3(1):32‐7. - PubMed
aa Zhilova 1986a {published data only}
-
- Zhilova GP, Ignat'eva GS, Orlov VA, Malikova EV, Maksakova VL. Results of a study of the effectiveness of simultaneous immunization against influenza with live and inactivated vaccines (1980 ‐ 1983). Voprosy Virusologii 1986;31(1):40‐4. - PubMed
aa Zhilova 1986b {published data only}
-
- Zhilova GP, Ignat'eva GS, Orlov VA, Malikova EV, Maksakova VL. Results of a study of the effectiveness of simultaneous immunization against influenza with live and inactivated vaccines (1980 ‐ 1983). Voprosy Virusologii 1986;31(1):40‐4. - PubMed
ab Atmar 1990 {published data only}
-
- Atmar RL, Bloom K, Keitel W, Couch RB, Greenberg SB. Effect of live attenuated, cold recombinant (CR) influenza virus vaccines on pulmonary function in healthy and asthmatic adults. Vaccine 1990;8(3):217‐24. - PubMed
ab Betts 1977a {published data only}
-
- Betts RF, Douglas RG Jr, Roth FK, Little JW 3rd. Efficacy of live attenuated influenza A/Scotland/74 (H3N2) virus vaccine against challenge with influenza A/Victoria/3/75 (H3N2) virus. Journal of Infectious Diseases 1977;136(6):746‐53. - PubMed
ab Boyce 2000 {published data only}
-
- Boyce TG, Hsu HH, Sannella EC, Coleman‐Dockery SD, Baylis E, Zhu Y, et al. Safety and immunogenicity of adjuvanted and unadjuvanted subunit influenza vaccines administered intranasally to healthy adults. Vaccine 2000;19(2‐3):217‐26. - PubMed
ab Caplan 1977 {published data only}
-
- Caplan ES, Hughes TP, O'Donnel S, Levine MM, Hornick RB. Reactogenicity and immunogenicity of parenteral monovalent influenza A/Victoria/3/75 (H3N2) virus vaccine in healthy adults. Journal of Infectious Diseases 1977;136(Suppl):484‐90. - PubMed
ab El'shina 1996 {published data only}
-
- El'shina GA, Masalin IM, Shervali VI, Gorbunov MA, Lonskaia NI, Agafonova LV, et al. The trivalent polymer‐subunit influenza vaccine Grippol studied in a controlled epidemiological trial. Voenno‐Meditsinskii Zhurnal 1996;317(8):57‐60. - PubMed
ab Evans 1976 {published data only}
ab Forsyth 1967 {published data only}
ab Goodeve 1983 {published data only}
ab Hrabar 1977 {published data only}
-
- Hrabar A, Vodopija I, Andre FE, Mitchell JR, Maassab HF, Hennessy AV, et al. A placebo‐controlled dose‐response study of the reactogenicity and immunogenicity of a cold‐adapted recombinant A/Victoria/3/75 (H3N2) live influenza virus candidate vaccine in healthy volunteers. Developments in Biological Standardization 1977;39:53‐60. - PubMed
ab Keitel 1993a {published data only}
-
- Keitel WA, Couch RB, Quarles JM, Cate TR, Baxter B, Maassab HF. Trivalent attenuated cold‐adapted influenza virus vaccine: reduced viral shedding and serum antibody responses in susceptible adults. Journal of Infectious Diseases 1993;167(2):305‐11. - PubMed
ab Keitel 1993b {published data only}
-
- Keitel WA, Couch RB, Quarles JM, Cate TR, Baxter B, Maassab HF. Trivalent attenuated cold‐adapted influenza virus vaccine: reduced viral shedding and serum antibody responses in susceptible adults. Journal of Infectious Diseases 1993;167(2):305‐11. - PubMed
ab Langley 2005 {published data only}
-
- Langley JM, Halperin SA, McNeil S, Smith B, Jones T, Burt D, et al. Safety and immunogenicity of a Proteosome‐trivalent inactivated influenza vaccine, given nasally to healthy adults. Vaccine 2005;24(10):1601‐8. - PubMed
ab Lauteria 1974 {published data only}
-
- Lauteria SF, Kantzler GB, High PC, Lee JD, Waldman RH. An attenuated influenza virus vaccine: reactogenicity, transmissibility, immunogenicity, and protective efficacy. Journal of Infectious Diseases 1974;130(4):380‐3. - PubMed
ab Miller 1977 {published data only}
-
- Miller LW, Togo Y, Hornick RB. Clinical and serologic effects of live attenuated serum inhibitor‐resistant influenza B vaccine in seronegative adults. Journal of Medical Virology 1977;1(3):193‐9. - PubMed
ab Pyrhönen 1981 {published data only}
-
- Pyrhönen S, Suni J, Romo M. Clinical trial of a subunit influenza vaccine. Scandinavian Journal of Infectious Diseases 1981;13:95‐9. - PubMed
ab Reeve 1982 {published data only}
-
- Reeve P, Pibermann M, Bachmayer H, Liehl E, Moritz A, Ganzinger U, et al. Studies in man with a cold‐recombinant live influenza B virus vaccine. Journal of Medical Virology 1982;9(1):1‐9. - PubMed
ab Rocchi 1979a {published data only}
ab Rocchi 1979b {published data only}
ab Saxen 1999 {published data only}
-
- Saxen H, Virtanen M. Randomized, placebo‐controlled double blind study on the efficacy of influenza immunization on absenteeism of health care workers. Pediatric Infectious Disease Journal 1999;18(9):779‐83. - PubMed
ab Scheifele 2003 {published data only}
-
- Scheifele DW, Duval B, Russell ML, Warrington R, DeSerres G, Skowronski DM, et al. Ocular and respiratory symptoms attributable to inactivated split influenza vaccine: evidence from a controlled trial involving adults. Clinical Infectious Diseases 2003;36(7):850‐7. - PubMed
ab Spencer 1977 {published data only}
bb Dauvilliers 2013 {published data only}
-
- Dauvilliers Y, Arnulf I, Lecendreux M, Monaca Charley C, Franco P, Drouot X, et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain 2013;139(Pt 8):2486‐96. - PubMed
bb DeStefano 2003 {published data only}
-
- DeStefano F, Verstraeten T, Jackson LA, Okoro CA, Benson P, Black SB, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Archives of Neurology 2003;60(4):504‐9. - PubMed
bb Dieleman 2011a {published data only}
bb Dieleman 2011b {published data only}
bb Dieleman 2011c {published data only}
bb Dieleman 2011d {published data only}
bb Dieleman 2011e {published data only}
bb Galeotti 2013 {published data only}
bb Garbe 2012 {published data only}
-
- Garbe E, Andersohn F, Bronder E, Klimpel A, Thomae M, Kurtal H. Association between drug use and acute immune thrombocytopenia in adults: results from the Berlin case‐control surveillance study. Pharmacoepidemiology and Drug Safety 2011;20:S147.
-
- Garbe E, Andersohn F, Bronder E, Salama A, Klimpel A, Thomae M, et al. Drug‐induced immune thrombocytopaenia: results from the Berlin Case‐Control Surveillance Study. European Journal of Clinical Pharmacology 2012;68(5):821‐32. - PubMed
bb Grimaldi‐Bensouda 2011 {published data only}
-
- Grimaldi‐Bensouda L, Alpérovitch A, Besson G, Vial C, Cuisset JM, Papeix C, et al. Guillain‐Barre syndrome, influenzalike illnesses, and influenza vaccination during seasons with and without circulating A/H1N1 viruses. American Journal of Epidemiology 2011;174(3):326‐35. - PubMed
bb Grimaldi‐Bensouda 2012 {published data only}
-
- Grimaldi‐Bensouda L, Michel M, Aubrun E, Leighton P, Viallard JF, Adoue D, et al. A case‐control study to assess the risk of immune thrombocytopenia associated with vaccines. Blood 2012;120(25):4938‐44. - PubMed
-
- Grimaldi‐Bensouda L, Michel M, Viallard J‐F, Adoue D, Magy‐Bertrand N, Khellaf M, et al. A multicenter case‐control prospective study to assess the risk of immune thrombocytopenia (ITP) associated with vaccines in adults using the PGRx‐ITP registry. Blood 2011;118(21):1169.
bb Hernan 2004 {published data only}
-
- Hernan MA, Jick SS, Olek MJ, Jick H. Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study. Neurology 2004;63(5):838‐42. - PubMed
bb MacIntyre 2013 {published data only}
bb Mastrangelo 2000 {published data only}
-
- Mastrangelo G, Rossi CR, Pfahlberg A, Marzia V, Barba A, Baldo M, et al. Is there a relationship between influenza vaccinations and risk of melanoma? A population‐based case‐control study. European Journal of Epidemiology 2000;16(9):777‐82. - PubMed
bb Mutsch 2004 {published data only}
-
- Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. New England Journal of Medicine 2004;350(9):896‐903. - PubMed
bb Payne 2006 {published data only}
-
- Payne DC, Rose CE Jr, Kerrison J, Aranas A, Duderstadt S, McNeil MM. Anthrax vaccination and risk of optic neuritis in the United States military, 1998‐2003. Archives of Neurology 2006;63(6):871‐5. - PubMed
bb Ray 2011 {published data only}
-
- Ray P, Black S, Shinefield H, Dillon A, Carpenter D, Lewis E, et al. Vaccine Safety Datalink Team. Risk of rheumatoid arthritis following vaccination with tetanus, influenza and hepatitis B vaccines among persons 15‐59 years of age. Vaccine 2011;29(38):6592‐7. - PubMed
bb Rouleau 2014 {published data only}
-
- Rouleau I, Serres G, Skowronski DM, Drolet JP, Lemire C, Toth E, et al. Risk factors associated with anaphylaxis and other allergic‐like events following receipt of 2009 monovalent AS03‐adjuvanted pandemic influenza vaccine in Quebec, Canada. Vaccine 2014;32(28):3480‐7. - PubMed
bb Siscovick 2000 {published data only}
-
- Siscovick DS, Raghunathan TE, Lin D, Weinmann S, Arbogast P, Lemaitre RN, et al. Influenza vaccination and the risk of primary cardiac arrest. American Journal of Epidemiology 2000;152(7):674‐7. - PubMed
bb Zorzon 2003 {published data only}
-
- Zorzon M, Zivadinov R, Nasuelli D, Dolfini P, Bosco A, Bratina A, et al. Risk factors of multiple sclerosis: a case‐control study. Neurological Sciences 2003;24(4):242‐7. - PubMed
cb Bardage 2011 {published data only}
cb Baxter 2012 {published data only}
-
- Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, et al. A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in adults 18‐49 years of age. Vaccine 2012;30(20):3053‐60. - PubMed
cb Kaplan 1982 {published data only}
-
- Kaplan JE, Katona P, Hurwitz ES, Schonberger LB. Guillain‐Barre syndrome in the United States, 1979‐1980 and 1980‐1981. Lack of an association with influenza vaccination. JAMA 1982;248(6):698‐700. - PubMed
cb Lasky 1998 {published data only}
-
- Lasky T, Terracciano GJ, Magder L, Koski CL, Ballesteros M, Nash D, et al. The Guillain‐Barre syndrome and the 1992‐1993 and 1993‐1994 influenza vaccines. New England Journal of Medicine 1998;339(25):1797‐802. - PubMed
cb Moro 2013 {published data only}
-
- Moro ML, Nobilio L, Voci C, Mario S, Candela S, Magrini N. A population based cohort study to assess the safety of pandemic influenza vaccine Focetria in Emilia‐Romagna region, Italy ‐ part two. Vaccine 2013;31(10):1438‐46. - PubMed
cb O'Flanagan 2014 {published data only}
-
- O'Flanagan D, Barret AS, Foley M, Cotter S, Bonner C, Crowe C, et al. Investigation of an association between onset of narcolepsy and vaccination with pandemic influenza vaccine, Ireland April 2009‐December 2010. Eurosurveillance 2014;19(17):15‐25. - PubMed
cb Persson 2014 {published data only}
-
- Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune‐related diseases, including narcolepsy, after vaccination with Pandemrix: a population‐ and registry‐based cohort study with over 2 years of follow‐up. Journal of Internal Medicine 2014;275(2):172‐90. - PubMed
cb Ray 2011 {published data only}
-
- Ray P, Black S, Shinefield H, Dillon A, Carpenter D, Lewis E, et al. Risk of rheumatoid arthritis following vaccination with tetanus, influenza and hepatitis B vaccines among persons 15‐59 years of age. Vaccine 2011;29(38):6592‐7. - PubMed
cb Shonberger 1979 {published data only}
-
- Schonberger LB, Bregman DJ, Sullivan‐Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain‐Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976 ‐ 1977. American Journal of Epidemiology 1979;110(2):105‐23. - PubMed
paa Ma 2014 {published data only}
paa Madhi 2014 {published data only}
-
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. Influenza vaccination of pregnant women and protection of their infants. New England Journal of Medicine 2014;371(10):918‐31. - PubMed
pba Benowitz 2010 {published data only}
pba Poehling 2011 {published data only}
pbb Irving 2013 {published data only}
-
- Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, Destefano F, et al. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstetrics and Gynecology 2013;121(1):159‐65. - PubMed
pca Ahrens 2014 {published data only}
pca Black 2004 {published data only}
-
- Black SB, Shinefield HR, France EK, Fireman BH, Blatt ST, Shay D. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. American Journal of Perinatology 2004;21(6):333‐9. - PubMed
pca Eick 2011 {published data only}
-
- Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, Santosham M, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Archives of Pediatrics and Adolescent Medicine 2011;165(2):104‐11. - PubMed
pca France 2006 {published data only}
-
- France EK, Smith‐Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Archives of Pediatrics and Adolescent Medicine 2006;160(12):1277‐83. - PubMed
pca Hulka 1964 {published data only}
-
- Hulka JF. Effectiveness of polyvalent influenza vaccine in pregnancy. Report of a controlled study during an outbreak of Asian influenza. Obstetrics and Gynecology 1964;23:830‐7. - PubMed
pca Munoz 2005 {published data only}
-
- Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. American Journal of Obstetrics and Gynecology 2005;192(4):1098‐106. - PubMed
pca Yamada 2012 {published data only}
-
- Yamada T, Yamada T, Morikawa M, Cho K, Endo T, Sato SS, et al. Pandemic (H1N1) 2009 in pregnant Japanese women in Hokkaido. Journal of Obstetrics and Gynaecology Research 2012;38(1):130‐6. - PubMed
pcb Beau 2014 {published data only}
-
- Beau AB, Hurault‐Delarue C, Vidal S, Guitard C, Vayssiere C, Petiot D. Pandemic A/H1N1 influenza vaccination during pregnancy: a comparative study using the EFEMERIS database. Vaccine 2014;32(11):1254‐8. - PubMed
pcb Cantu 2013 {published data only}
-
- Cantu J, Biggio J, Jauk V, Wetta L, Andrews W, Tita A. Selective uptake of influenza vaccine and pregnancy outcomes. Journal of Maternal‐Fetal and Neonatal Medicine 2013;26(12):1207‐11. - PubMed
pcb Chambers 2013 {published data only}
-
- Chambers CD, Johnson D, Xu R, Luo Y, Louik C, Mitchell AA, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine 2013;31(44):5026‐32. - PubMed
pcb Cleary 2014 {published data only}
-
- Cleary BJ, Rice U, Eogan M, Metwally N, McAuliffe F. 2009 A/H1N1 influenza vaccination in pregnancy: uptake and pregnancy outcomes ‐ a historical cohort study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;178:163‐8. - PubMed
pcb Deinard 1981 {published data only}
-
- Deinard AS, Ogburn P Jr. A/NJ/8/76 influenza vaccination program: effects on maternal health and pregnancy outcome. American Journal of Obstetrics and Gynecology 1981;140(3):240‐5. - PubMed
pcb Dodds 2012 {published data only}
-
- Dodds L, Macdonald N, Scott J, Spencer A, Allen VM, McNeil S. The association between influenza vaccine in pregnancy and adverse neonatal outcomes. Journal of Obstetrics and Gynaecology Canada 2012;34(8):714‐20. - PubMed
pcb Fell 2012 {published data only}
pcb Håberg 2013 {published data only}
pcb Heikkinen 2012 {published data only}
-
- Heikkinen T, Young J, Beek E, Franke H, Verstraeten T, Weil JG, et al. Safety of MF59‐adjuvanted A/H1N1 influenza vaccine in pregnancy: a comparative cohort study. American Journal of Obstetrics & Gynecology 2012;207(3):177.e1‐8. - PubMed
pcb Källén 2012 {published data only}
-
- Källén B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix during pregnancy and delivery outcome: a Swedish register study. British Journal of Obstetrics and Gynaecology 2012;119(13):1583‐90. - PubMed
pcb Launay 2012 {published data only}
pcb Lin 2012 {published data only}
-
- Lin TH, Lin SY, Lin CH, Lin RI, Lin HC, Chiu TH, et al. AdimFlu‐S influenza A (H1N1) vaccine during pregnancy: the Taiwanese Pharmacovigilance Survey. Vaccine 2012;30(16):2671‐5. - PubMed
pcb Louik 2013 {published data only}
-
- Louik C, Ahrens K, Kerr S, Pyo J, Chambers C, Jones KL, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: exposure prevalence, preterm delivery, and specific birth defects. Vaccine 2013;31(44):5033‐40. - PubMed
pcb Ludvigsson 2013 {published data only}
-
- Ludvigsson JF, Zugna D, Cnattingius S, Richiardi L, Ekbom A, Ortqvist A, et al. Influenza H1N1 vaccination and adverse pregnancy outcome. European Journal of Epidemiology 2013;28(7):579‐88. - PubMed
pcb Nordin 2013 {published data only}
-
- Nordin JD, Kharbanda EO, Benitez GV, Nichol K, Lipkind H, Naleway A, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstetrics and Gynecology 2013;121(3):519‐25. - PubMed
pcb Nordin 2014 {published data only}
-
- Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F. Maternal influenza vaccine and risks for preterm or small for gestational age birth. Journal of Pediatrics 2014;164(5):1051‐7.e2. - PubMed
pcb Omer 2011 {published data only}
pcb Oppermann 2012 {published data only}
-
- Oppermann M, Fritzsche J, Weber‐Schoendorfer C, Keller‐Stanislawski B, Allignol A, Meister R, et al. A(H1N1)v2009: a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine 2012;30(30):4445‐52. - PubMed
pcb Pasternak 2012 {published data only}
-
- Pasternak B, Svanström H, Mølgaard‐Nielsen D, Krause TG, Emborg HD, Melbye M, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A (H1N1) vaccine during pregnancy. JAMA 2012;308(2):165‐74. - PubMed
pcb Richards 2013 {published data only}
-
- Richards JL, Hansen C, Bredfeldt C, Bednarczyk RA, Steinhoff MC, Adjaye‐Gbewonyo D, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clinical Infectious Diseases 2013;56(9):1216‐22. - PMC - PubMed
pcb Rubinstein 2013 {published data only}
pcb Sheffield 2012 {published data only}
-
- Sheffield JS, Greer LG, Rogers VL, Roberts SW, Lytle H, McIntire DD, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstetrics and Gynecology 2012;120(3):532‐7. - PubMed
pcb Toback 2012 {published data only}
References to studies excluded from this review
ab López‐Macías 2011a {published data only}
-
- López‐Macías C, Ferat‐Osorio E, Tenorio‐Calvo A, Isibasi A, Talavera J, Arteaga‐Ruiz O, et al. Safety and immunogenicity of a virus‐like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomised, placebo‐controlled trial of adults in Mexico. Vaccine 2011;29(44):7826‐34. - PMC - PubMed
ab López‐Macías 2011b {published data only}
-
- López‐Macías C, Ferat‐Osorio E, Tenorio‐Calvo A, Isibasi A, Talavera J, Arteaga‐Ruiz O, et al. Safety and immunogenicity of a virus‐like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomised, placebo‐controlled trial of adults in Mexico. Vaccine 2011;29(44):7826‐34. - PMC - PubMed
ab Mallory 2010 {published data only}
ab Plennevaux 2010 {published data only}
-
- Plennevaux E, Sheldon E, Blatter M, Reeves‐Hoché MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 2010;375(9708):41‐8. - PubMed
ab Precioso 2011 {published data only}
-
- Precioso AR, Miraglia JL, Campos LM, Goulart AC, Timenetsky Mdo C, Cardoso MR, et al. A phase I randomised, double‐blind, controlled trial of 2009 influenza A (H1N1) inactivated monovalent vaccines with different adjuvant systems. Vaccine 2011;29(48):8974‐81. - PubMed
ab Treanor 2010 {published data only}
-
- Treanor JJ, Taylor DN, Tussey L, Hay C, Nolan C, Fitzgerald T, et al. Safety and immunogenicity of a recombinant hemagglutinin influenza‐flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine 2010;28(52):8268‐74. - PubMed
ab Turley 2011 {published data only}
-
- Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e‐flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 2011;29(32):5145‐52. - PubMed
ab Wacheck 2010 {published data only}
-
- Wacheck V, Egorov A, Groiss F, Pfeiffer A, Fuereder T, Hoeflmayer D, et al. A novel type of influenza vaccine: safety and immunogenicity of replication‐deficient influenza virus created by deletion of the interferon antagonist NS1. Journal of Infectious Diseases 2010;201(3):354‐62. - PubMed
Al‐Dabbagh 2013 {published data only}
-
- Al‐Dabbagh M, Lapphra K, Scheifele DW, Halperin SA, Langley JM, Cho P, et al. Elevated inflammatory mediators in adults with oculo‐respiratory syndrome following influenza immunization: a public health agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) Study. Clinical and Vaccine Immunology 2013;20(8):1108‐14. - PMC - PubMed
Ambrosch 1976 {published data only}
-
- Ambrosch F, Balluch H. Studies of the non‐specific clinical effectiveness of influenza vaccination. Laryngologie, Rhinologie, Otologie 1976;55:57‐61. - PubMed
Ambrose 2012 {published data only}
-
- Ambrose CS, Wu X. The safety and effectiveness of self‐administration of intranasal live attenuated influenza vaccine in adults. Vaccine 2013;31(6):857‐60. - PubMed
Andersson 2015 {published data only}
-
- Andersson L. Response on the author's reply to the letter to the editor: Contradictory data on type 1 diabetes in a recently published article "Risks of neurological and immune‐related diseases, including narcolepsy, after vaccination with Pandemrix". Journal of Internal Medicine 2015;277(2):272‐3. - PubMed
Aoki 1986 {published data only}
Arnou 2010 {published data only}
-
- Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18‐60 years: randomized, controlled, phase III trial. Human Vaccines 2010;6(4):346‐54. - PubMed
Atmar 1995 {published data only}
-
- Atmar RL, Keitel WA, Cate TR, Quarles JM, Couch RB. Comparison of trivalent cold‐adapted recombinant (CR) influenza virus vaccine with monovalent CR vaccines in healthy unselected adults. Journal of Infectious Diseases 1995;172(1):253‐7. - PubMed
Atmar 2011 {published data only}
Atsmon 2012 {published data only}
-
- Atsmon J, Kate‐Ilovitz E, Shaikevich D, Singer Y, Volokhov I, Haim KY, et al. Safety and immunogenicity of multimeric‐001 ‐ a novel universal influenza vaccine. Journal of Clinical Immunology 2012;32(3):595‐603. - PubMed
Ausseil 1999 {published data only}
-
- Ausseil F. Immunization against influenza among working adults: the Philippines experience. Vaccine 1999;17(Suppl 1):59‐62. - PubMed
Banzhoff 2001 {published data only}
-
- Banzhoff A, Kaniok W, Muszer A. Effectiveness of an influenza vaccine used in Poland in the 1998‐1999 influenza season. Immunological Investigations 2001;30(2):103‐13. - PubMed
Baxter 2010 {published data only}
-
- Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine 2010;28(45):7267‐72. - PubMed
Baxter 2011 {published data only}
-
- Baxter R, Patriarca PA, Ensor K, Izikson R, Goldenthal KL, Cox MM. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus‐expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50‐64 years of age. Vaccine 2011;29(12):2272‐8. - PubMed
Baxter 2012 {published data only}
-
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP. Recurrent Guillain‐Barre syndrome following vaccination. Clinical Infectious Diseases 2012;54(6):800‐4. - PubMed
Baxter 2013 {published data only}
-
- Baxter R, Bakshi N, Fireman B, Lewis E, Ray P, Vellozzi C, et al. Lack of association of Guillain‐Barre syndrome with vaccinations. Clinical Infectious Diseases 2013;57(2):197‐204. - PubMed
Belongia 2009 {published data only}
-
- Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004‐2005 season to the 2006‐2007 season. Journal of Infectious Diseases 2009;199(2):159‐67. - PubMed
Belshe 2001 {published data only}
Benke 2004 {published data only}
-
- Benke G, Abramson M, Raven J, Thien FCK, Walters EH. Asthma and vaccination history in a young adult cohort. Australian and New Zealand Journal of Public Health 2004;28(4):336‐8. - PubMed
Beran 2013 {published data only}
Betts 1977b {published data only}
-
- Betts RF, Douglas RG Jr. Comparative study of reactogenicity and immunogenicity of influenza A/New Jersey/8/76 (Hsw1N1) virus vaccines in normal volunteers. Journal of Infectious Diseases 1977;136(Suppl):443‐9. - PubMed
Beyer 1996 {published data only}
-
- Beyer WEP, Palache AM, Kerstens R, Masurel N. Gender differences in local and systemic reactions to inactivated influenza vaccine, established by a meta‐analysis of fourteen independent studies. European Journal of Clinical Microbiology & Infectious Diseases 1996;15(1):65‐70. - PubMed
Carlson 1979 {published data only}
-
- Carlson AJ, Davidson WL, McLean AA, Vella PP, Weibel RE, Woodhour AF, et al. Pneumococcal vaccine: dose, revaccination, and coadministration with influenza vaccine. Proceedings of the Society for Experimental Biology and Medicine 1979;161(4):558‐63. - PubMed
Cate 1977 {published data only}
-
- Cate TR, Couch RB, Kasel JA, Six HR. Clinical trials of monovalent influenza A/New Jersey/76 virus vaccines in adults: reactogenicity, antibody response, and antibody persistence. Journal of Infectious Diseases 1977;136(Suppl):450‐5. - PubMed
Chavant 2013 {published data only}
-
- Chavant F, Ingrand I, Jonville‐Bera AP, Plazanet C, Gras‐Champel V, Lagarce L, et al. The PREGVAXGRIP study: a cohort study to assess foetal and neonatal consequences of in utero exposure to vaccination against A (H1N1) v2009 influenza. Drug Safety 2013;36(6):455‐65. - PubMed
Chichester 2012 {published data only}
-
- Chichester JA, Jones RM, Green BJ, Stow M, Miao F, Moonsammy G, et al. Safety and immunogenicity of a plant‐produced recombinant hemagglutinin‐based influenza vaccine (HAI‐05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: a phase 1 randomised, double‐blind, placebo‐controlled, dose‐escalation study in healthy adults. Viruses 2012;4(11):3227‐44. - PMC - PubMed
Chlibek 2002 {published data only}
-
- Chlibek R, Beran J, Splino M. Effectiveness of influenza vaccination in healthy adults ‐ a fourfold decrease in influenza morbidity during one influenza season. Epidemiologie, Mikrobiologie, Imunologie 2002;51(2):47‐51. - PubMed
Choe 2011a {published data only}
-
- Choe YJ, Cho H, Song KM, Kim JH, Han OP, Kwon YH, et al. Active surveillance of adverse events following immunization against pandemic influenza A (H1N1) in Korea. Japanese Journal of Infectious Diseases 2011;64(4):297‐303. - PubMed
Choe 2011b {published data only}
-
- Choe YJ, Cho H, Bae GR, Lee JK. Guillain‐Barre syndrome following receipt of influenza A (H1N1) 2009 monovalent vaccine in Korea with an emphasis on Brighton Collaboration case definition. Vaccine 2011;29(11):2066‐70. - PubMed
Choe 2011c {published data only}
-
- Choe YJ, Cho H, Kim SN, Bae GR, Lee JK. Serious adverse events following receipt of trivalent inactivated influenza vaccine in Korea, 2003‐2010. Vaccine 2011;29(44):7727‐32. - PubMed
Chou 2007 {published data only}
-
- Chou CH, Liou WP, Hu KI, Loh CH, Chou CC, Chen YH. Bell’s palsy associated with influenza vaccination: two case reports. Vaccine 2007;25:2839–41. - PubMed
Clover 1991 {published data only}
-
- Clover RD, Crawford S, Glezen WP, Taber LH, Matson CC, Couch RB. Comparison of heterotypic protection against influenza A/Taiwan/86 (H1N1) by attenuated and inactivated vaccines to A/Chile/83‐like viruses. Journal of Infectious Diseases 1991;163(2):300‐4. - PubMed
Confavreux 2001 {published data only}
-
- Confavreux C, Suissa S, Saddier P, Bourdes V, Vukusic S. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. New England Journal of Medicine 2001;344(5):319‐26. - PubMed
Conlin 2013 {published data only}
-
- Conlin AMS, Bukowinski AT, Sevick CJ, DeScisciolo C, Crum‐Cianflone NF. Safety of the pandemic H1N1 influenza vaccine among pregnant U.S. military women and their newborns. Obstetrics and Gynecology 2013;121(3):511‐8. - PubMed
Couch 2012 {published data only}
Das Gupta 2002 {published data only}
-
- Das Gupta R, Guest JF. A model to estimate the cost benefit of an occupational vaccination programme for influenza with Influvac in the UK. Pharmacoeconomics 2002;20(7):475‐84. - PubMed
Davidson 2011 {published data only}
Davies 1972 {published data only}
-
- Davies JE, Howell RW, Meichen FW. A clinical trial of inhaled inactivated influenza vaccine. British Journal of Clinical Practice 1972;26(10):469‐71. - PubMed
Davies 1973 {published data only}
-
- Davies JE, Howell RH, Meichen FW. A clinical trial of inhaled inactivated influenza vaccine. British Journal of General Practice 1970;27(6):219‐21. - PubMed
De Serres 2003a {published data only}
-
- Serres GI, Boulianne N, Duval B, Rochette L, Grenier JL, Roussel R, et al. Oculo‐respiratory syndrome following influenza vaccination: evidence for occurrence with more than one influenza vaccine. Vaccine 2003;21(19‐20):2346‐53. - PubMed
De Serres 2003b {published data only}
-
- Serres GI, Grenier JL, Toth E, Menard S, Roussel R, Tremblay M, et al. The clinical spectrum of the oculo‐respiratory syndrome after influenza vaccination. Vaccine 2003;21(19‐20):2354‐61. - PubMed
De Serres 2004 {published data only}
-
- Serres G, Skowronski DM, Guay M, Rochette L, Jacobsen K, Fuller T, et al. Recurrence risk of oculorespiratory syndrome after influenza vaccination: randomized controlled trial of previously affected persons. Archives of Internal Medicine 2004;164(20):2266‐72. - PubMed
De Wals 2012 {published data only}
-
- Wals P, Deceuninck G, Toth E, Boulianne N, Brunet D, Boucher RM, et al. Risk of Guillain‐Barre syndrome following H1N1 influenza vaccination in Quebec. JAMA 2012;308(2):175‐81. - PubMed
Dolin 1977 {published data only}
-
- Dolin R, Wise TG, Mazur MH, Tuazon CU, Ennis FA. Immunogenicity and reactogenicity of influenza A/New Jersey/76 virus vaccines in normal adults. Journal of Infectious Diseases 1977;136(Suppl):435‐42. - PubMed
Dominguez 2012 {published data only}
-
- Dominguez A, Castilla J, Godoy P, Delgado‐Rodriguez M, Martin V, Saez M, et al. Effectiveness of pandemic and seasonal influenza vaccines in preventing pandemic influenza‐associated hospitalization. Vaccine 2012;30(38):5644‐50. - PubMed
Duffy 2014 {published data only}
Eames 2012 {published data only}
-
- Eames KT, Brooks‐Pollock E, Paolotti D, Perosa M, Gioannini C, Edmunds WJ. Rapid assessment of influenza vaccine effectiveness: analysis of an internet‐based cohort. Epidemiology and Infection 2012;140(7):1309‐15. - PubMed
Edmonson 1970 {published data only}
-
- Edmonson KW, Graham SM, Warburton MF. A clinical trial of influenza vaccine in Canberra. Medical Journal of Australia 1970;4:6‐13. - PubMed
Eick‐Cost 2012 {published data only}
El'shina 1998 {published data only}
-
- El'shina GA, Gorbunov MA, Shervarli VI, Lonskaia NI, Pavlova LI, Khaitov RM, et al. Evaluation of the effectiveness of influenza trivalent polymer subunit vaccine "Grippol". Zhumal Mikrobiologii Epidemiologii Immunobiologii 1998;3:40‐3. - PubMed
Englund 1993 {published data only}
-
- Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. Journal of Infectious Diseases 1993;168(3):647‐56. - PubMed
Finklea 1969 {published data only}
-
- Finklea JF, Sandifer SH, Peck FB, Manos JP. A clinical and serologic comparison of standard and purified bivalent inactivated influenza vaccines. Journal of Infectious Diseases 1969;120(6):708‐12. - PubMed
Fisher 2012 {published data only}
Foy 1981 {published data only}
-
- Foy HM, Cooney MK, Allan ID, Frost F, Blumhagen JM, Fox JP. Influenza B virus vaccines in children and adults: adverse reactions, immune response, and observations in the field. Journal of Infectious Diseases 1981;143(5):700‐6. - PubMed
Frank 1981 {published data only}
-
- Frank AL, Webster RG, Glezen WP, Cate TR. Immunogenicity of influenza A/USSR (H1N1) subunit vaccine in unprimed young adults. Journal of Medical Virology 1981;7(2):135‐42. - PubMed
Freestone 1976 {published data only}
-
- Freestone DS. Clinical trials with intranasally administered WRL 105 strain live influenza vaccine in volunteers. Developments in Biological Standardization 1976;33:207‐12. - PubMed
Gerstoft 2001 {published data only}
-
- Gerstoft J. Influenza vaccination of healthy adults. Ugeskrift for Laeger 2001;163(19):2615‐7. - PubMed
Greenbaum 2002 {published data only}
-
- Greenbaum E, Furst A, Kiderman A, Stewart B, Levy R, Schlesinger M, et al. Mucosal [SIgA] and serum [IgG] immunologic responses in the community after a single intra‐nasal immunization with a new inactivated trivalent influenza vaccine. Vaccine 2002;20(7‐8):1232‐9. - PubMed
Greene 2013 {published data only}
Gross 1999 {published data only}
-
- Gross PA, Sperber SJ, Donabedian A, Dran S, Morchel G, Cataruozolo P, et al. Paradoxical response to a novel influenza virus vaccine strain: the effect of prior immunization. Vaccine 1999;17(18):2284‐9. - PubMed
Grotto 1998 {published data only}
-
- Grotto I, Mandel Y, Green MS, Varsano N, Gdalevich M, Ashkenazi I, et al. Influenza vaccine efficacy in young, healthy adults. Clinical Infectious Diseases 1998;26(4):913‐7. - PubMed
Gruber 1994 {published data only}
-
- Gruber WC, Campbell PW, Thompson JM, Reed GW, Roberts B, Wright PF. Comparison of live attenuated and inactivated influenza vaccines in cystic fibrosis patients and their families: results of a 3‐year study. Journal of Infectious Diseases 1994;169(2):241‐7. - PubMed
Gwini 2011 {published data only}
-
- Gwini SM, Coupland CA, Siriwardena AN. The effect of influenza vaccination on risk of acute myocardial infarction: self‐controlled case‐series study. Vaccine 2011;29(6):1145‐9. - PubMed
Haber 2004 {published data only}
-
- Haber P, DeStefano F, Angulo FJ, Iskander J, Shadomy SV, Weintraub E, et al. Guillain‐Barre syndrome following influenza vaccination. JAMA 2004;292(20):2478‐81. - PubMed
Haigh 1973 {published data only}
-
- Haigh W, Howell RW, Meichen FW. A comparative trial of influenza immunization by inhalation and hypojet method. The Practitioner 1973;211:365‐70. - PubMed
Halperin 2002 {published data only}
-
- Halperin SA, Smith B, Mabrouk T, Germain M, Trepanier P, Hassell T, et al. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture‐derived influenza vaccine in healthy adults, seniors, and children. Vaccine 2002;20(7‐8):1240‐7. - PubMed
Hambidge 2011 {published data only}
-
- Hambidge SJ, Ross C, McClure D, Glanz J. Trivalent inactivated influenza vaccine is not associated with sickle cell hospitalizations in adults from a large cohort. Vaccine 2011;29(46):8179‐81. - PubMed
Heinonen 1973 {published data only}
-
- Heinonen OP, Shapiro S, Monson RR, Hartz SC, Rosenberg L, Slone D. Immunization during pregnancy against poliomyelitis and influenza in relation to childhood malignancy. International Journal of Epidemiology 1973;2(3):229‐35. - PubMed
Hellenbrand 2012 {published data only}
-
- Hellenbrand W, Jorgensen P, Schweiger B, Falkenhorst G, Nachtnebel M, Greutelaers B, et al. Prospective hospital‐based case‐control study to assess the effectiveness of pandemic influenza A(H1N1)pdm09 vaccination and risk factors for hospitalization in 2009‐2010 using matched hospital and test‐negative controls. BMC Infectious Diseases 2012;12:127. - PMC - PubMed
Hobson 1970 {published data only}
Hobson 1973 {published data only}
Hoskins 1973 {published data only}
-
- Hoskins TW, Davies JR, Allchin A, Miller CL, Pollock TM. Controlled trial of inactivated influenza vaccine containing the A/Hong Kong strain during an outbreak of influenza due to the A/England/42/72 strain. Lancet 1973;2(7821):116‐20. - PubMed
Hoskins 1976 {published data only}
-
- Hoskins TW, Davies JR, Smith AJ, Allchin A, Miller CL, Pollock TM. Influenza at Christ's Hospital: March, 1974. Lancet 1976;1(7951):105‐8. - PubMed
Hoskins 1979 {published data only}
-
- Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet 1979;1(8106):33‐5. - PubMed
Howell 1967 {published data only}
Huang 2011 {published data only}
Hurwitz 1983 {published data only}
-
- Hurwitz ES, Holman RC, Nelson DB, Schonberger LB. National surveillance for Guillain‐Barré syndrome: January 1978‐March 1979. Neurology 1983;33(2):150‐7. - PubMed
Jackson 2011 {published data only}
Janjua 2012 {published data only}
-
- Janjua NZ, Skowronski DM, Serres G, Dickinson J, Crowcroft NS, Taylor M, et al. Estimates of influenza vaccine effectiveness for 2007‐2008 from Canada's sentinel surveillance system: cross‐protection against major and minor variants. Journal of Infectious Diseases 2012;205(12):1858‐68. - PubMed
Jianping 1999 {published data only}
-
- Jianping H, Xin F, Changshun L, Bo Z, Linxiu G, Wei X, et al. Assessment of effectiveness of Vaxigrip. Vaccine 1999;17(Suppl 1):57‐8. - PubMed
Jimenez‐Jorge 2012 {published data only}
-
- Jimenez‐Jorge S, Mateo S, Pozo F, Casas I, Garcia Cenoz M, Castilla J, et al. Early estimates of the effectiveness of the 2011/12 influenza vaccine in the population targeted for vaccination in Spain, 25 December 2011 to 19 February 2012. Euro Surveillance 2012;17(12):20129. - PubMed
Keitel 2001 {published data only}
-
- Keitel WA, Cate TR, Nino D, Huggins LL, Six HR, Quarles JM, et al. Immunization against influenza: comparison of various topical and parenteral regimens containing inactivated and/or live attenuated vaccines in healthy adults. Journal of Infectious Diseases 2001;183(2):329‐32. - PubMed
Kelly 2012 {published data only}
Khazeni 2009 {published data only}
Kiderman 2001 {published data only}
-
- Kiderman A, Furst A, Stewart B, Greenbaum E, Morag A, Zakay‐Rones Z. A double‐blind trial of a new inactivated, trivalent, intra‐nasal anti‐influenza vaccine in general practice: relationship between immunogenicity and respiratory morbidity over the winter of 1997‐98. Journal of Clinical Virology 2001;20(3):155‐61. - PubMed
Kim 2012 {published data only}
-
- Kim J‐H, Cho H‐Y, Hennessey KA, Lee HJ, Bae GR, Kim HC. Adverse events following immunization (AEFI) with the novel influenza A (H1N1) 2009 vaccine: findings from the national registry of all vaccine recipients and AEFI and the passive surveillance system in South Korea. Japanese Journal of Infectious Diseases 2012;65(2):99‐104. - PubMed
Kissling 2012 {published data only}
-
- Kissling E, Valenciano M. Early estimates of seasonal influenza vaccine effectiveness in Europe among target groups for vaccination: results from the I‐MOVE multicentre case‐control study, 2011/12. Euro Surveillance 2012;17(15):20146. - PubMed
Kunz 1977 {published data only}
-
- Kunz C, Hofmann H, Bachmayer H, Liehl E, Moritz AJ. Clinical trials with a new influenza subunit vaccine in adults and children. Developments in Biological Standardization 1977;39:297‐302. - PubMed
Langley 2004 {published data only}
Lavallee 2014 {published data only}
-
- Lavallee PC, Labreuche J, Fox KM, Lavados P, Mattle H, Steg PG, et al. Influenza vaccination and cardiovascular risk in patients with recent TIA and stroke. Neurology 2014;82(21):1905‐13. - PubMed
Lee 2011 {published data only}
-
- Lee GM, Greene SK, Weintraub ES, Baggs J, Kulldorff M, Fireman BH, et al. H1N1 and seasonal influenza vaccine safety in the Vaccine Safety Datalink project. American Journal of Preventive Medicine 2011;41(2):121‐8. - PubMed
Leeb 2011 {published data only}
-
- Leeb A, Carcione D, Richmond PC, Jacoby P, Effler PV. Reactogenicity of two 2010 trivalent inactivated influenza vaccine formulations in adults. Vaccine 2011;29(45):7920‐4. - PubMed
Leroux‐Roels 2010a {published data only}
-
- Leroux‐Roels I, Roman F, Forgus S, Maes C, Boever F, Drame M, et al. Priming with AS03 A‐adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non‐randomised extension of a double‐blind randomised primary study. Vaccine 2010;28(3):849‐57. - PubMed
Leroux‐Roels 2010b {published data only}
-
- Leroux‐Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, et al. Corrigendum to: "Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults" by Leroux‐Roels et al. [Vaccine 26 (2008) 6614‐6619]. Vaccine 2010;28(50):8033. - PubMed
Liem 1973 {published data only}
Lind 2014 {published data only}
-
- Lind A, Ramelius A, Olsson T, Arnheim‐Dahlstrom L, Lamb F, Khademi M, et al. A/H1N1 antibodies and TRIB2 autoantibodies in narcolepsy patients diagnosed in conjunction with the Pandemrix vaccination campaign in Sweden 2009‐2010. Journal of Autoimmunity 2014;50:99‐106. - PubMed
Liu 2012 {published data only}
Louik 2013 {published data only}
-
- Louik C, Chambers C, Jacobs D, Rice F, Johnson D, Mitchell AA. Influenza vaccine safety in pregnancy: can we identify exposures?. Pharmacoepidemiology and Drug Safety 2013;22(1):33‐9. - PubMed
Mackenzie 1975 {published data only}
Mackenzie 2012 {published data only}
-
- Mackenzie IS, Macdonald TM, Shakir S, Dryburgh M, Mantay BJ, Mcdonnell P, et al. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self‐reporting of serious adverse events and pregnancy outcomes. British Journal of Clinical Pharmacology 2012;73(5):801‐11. - PMC - PubMed
Mair 1974 {published data only}
Maynard 1968 {published data only}
-
- Maynard JE, Dull HB, Hanson ML, Feltz ET, Berger R, Hammes L. Evaluation of monovalent and polyvalent influenza vaccines during an epidemic of type A2 and B influenza. American Journal of Epidemiology 1968;87(1):148‐57. - PubMed
McCarthy 2004 {published data only}
-
- McCarthy MW, Kockler DR. Trivalent intranasal influenza vaccine, live. Annals of Pharmacotherapy 2004;38(12):2086‐93. - PubMed
Mendelman 2001 {published data only}
-
- Mendelman PM, Cordova J, Cho I. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold‐adapted (CAIV‐T) in healthy children and healthy adults. Vaccine 2001;19(17‐19):2221‐6. - PubMed
Merelli 2000 {published data only}
-
- Merelli E, Casoni F. Prognostic factors in multiple sclerosis: role of intercurrent infections and vaccinations against influenza and hepatitis B. Neurological Sciences 2000;21(4 Suppl 2):853‐6. - PubMed
Meyers 2003a {published data only}
-
- Meyers DG. Could influenza vaccination prevent myocardial infarction, stroke and sudden cardiac death?. American Journal of Cardiovascular Drugs 2003;3(4):241‐4. - PubMed
Meyers 2003b {published data only}
-
- Meyers DG. Myocardial infarction, stroke, and sudden cardiac death may be prevented by influenza vaccination. Current Atherosclerosis Reports 2003;5(2):146‐9. - PubMed
Micheletti 2011 {published data only}
-
- Micheletti F, Moretti U, Tridente G, Zanoni G. Consultancy and surveillance of post‐immunisation adverse events in the Veneto region of Italy for 1992‐2008. Human Vaccines 2011;7(Suppl):234‐9. - PubMed
Monto 2000 {published data only}
-
- Monto AS. Preventing influenza in healthy adults: the evolving story. JAMA 2000;284(13):1699‐701. - PubMed
Montplaisir 2014 {published data only}
Moro 2011 {published data only}
-
- Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. American Journal of Obstetrics & Gynecology 2011;205(5):473.e1‐9. - PMC - PubMed
Morris 1975 {published data only}
-
- Morris CA, Freestone DS, Stealey VM, Oliver PR. Recombinant WRL 105 strain live attenuated influenza vaccine. Immunogenicity, reactivity, and transmissibility. Lancet 1975;2(7927):196‐9. - PubMed
Mostow 1977 {published data only}
-
- Mostow SR, Eichkoff TC, Chelgren GA, Retailliau HF, Castle M. Studies of inactivated influenza virus vaccines in hospital employees: reactogenicity and absenteeism. Journal of Infectious Diseases 1977;136 Suppl:S533‐8. - PubMed
Muennig 2001 {published data only}
-
- Muennig PA, Khan K. Cost‐effectiveness of vaccination versus treatment of influenza in healthy adolescents and adults. Clinical Infectious Diseases 2001;33(11):1879‐85. - PubMed
Murray 1979 {published data only}
Nazareth 2013 {published data only}
Nichol 1996 {published data only}
-
- Nichol KL, Margolis KL, Lind A, Murdoch M, McFadden R, Hauge M, et al. Side effects associated with influenza vaccination in healthy working adults. Archives of Internal Medicine 1996;156(14):1546‐50. - PubMed
Nichol 1999b {published data only}
-
- Nichol KL. Clinical effectiveness and cost effectiveness of influenza vaccination among healthy working adults. Vaccine 1999;17(Suppl 1):67‐73. - PubMed
Nichol 2001 {published data only}
-
- Nichol KL. Live attenuated influenza virus vaccines: new options for the prevention of influenza. Vaccine 2001;19(31):4373‐7. - PubMed
Nichol 2003 {published data only}
-
- Nichol KL, Mallon KP, Mendelman PM. Cost benefit of influenza vaccination in healthy, working adults: an economic analysis based on the results of a clinical trial of trivalent live attenuated influenza virus vaccine. Vaccine 2003;21(17‐8):2207‐17. - PubMed
Nichol 2004 {published data only}
-
- Nichol KL, Mendelman P. Influence of clinical case definitions with differing levels of sensitivity and specificity on estimates of the relative and absolute health benefits of influenza vaccination among healthy working adults and implications for economic analyses. Virus Research 2004;103(1‐2):3‐8. - PubMed
Omon 2011 {published data only}
-
- Omon E, Damase‐Michel C, Hurault‐Delarue C, Lacroix I, Montastruc JL, Oustric S, et al. Non‐adjuvanted 2009 influenza A (H1N1)v vaccine in pregnant women: the results of a French prospective descriptive study. Vaccine 2011;29(52):9649‐54. - PubMed
Petrie 2011 {published data only}
Phillips 2013 {published data only}
-
- Phillips CJ, Woolpert T, Sevick C, Faix D, Blair PJ, Crum‐Cianflone NF. Comparison of the effectiveness of trivalent inactivated influenza vaccine and live, attenuated influenza vaccine in preventing influenza‐like illness among US military service members, 2006‐2009. Clinical Infectious Diseases 2013;56(1):11‐9. - PubMed
Phonrat 2013 {published data only}
-
- Phonrat B, Pitisuttithum P, Chamnanchanunt S, Puthavathana P, Ngaosuwankul N, Louisirirotchanakul S, et al. Safety and immune responses following administration of H1N1 live attenuated influenza vaccine in Thais. Vaccine 2013;31(11):1503‐9. - PubMed
Pleguezuelos 2012 {published data only}
-
- Pleguezuelos O, Robinson S, Stoloff GA, Caparrós‐Wanderley W. Synthetic influenza vaccine (FLU‐v) stimulates cell mediated immunity in a double‐blind, randomised, placebo‐controlled Phase I trial. Vaccine 2012;30(31):4655‐60. - PubMed
Puig‐Barbera 2012 {published data only}
-
- Puig‐Barbera J, Diez‐Domingo J, Arnedo‐Pena A, Ruiz‐Garcia M, Perez‐Vilar S, Mico‐Esparza JL, et al. Effectiveness of the 2010‐2011 seasonal influenza vaccine in preventing confirmed influenza hospitalizations in adults: a case‐case comparison, case‐control study. Vaccine 2012;30(39):5714‐20. - PMC - PubMed
Puleston 2010 {published data only}
-
- Puleston RL, Bugg G, Hoschler K, Konje J, Thornton J, Stephenson I, et al. Observational study to investigate vertically acquired passive immunity in babies of mothers vaccinated against H1N1v during pregnancy. Health Technology Assessment 2010;14(55):1‐82. - PubMed
Pyhala 2001 {published data only}
-
- Pyhala R, Haanpaa M, Kleemola M, Tervahauta R, Visakorpi R, Kinnunen L. Acceptable protective efficacy of influenza vaccination in young military conscripts under circumstances of incomplete antigenic and genetic match. Vaccine 2001;19(23‐4):3253‐60. - PubMed
Reynales 2012 {published data only}
-
- Reynales H, Astudillo P, Valliere S, Hatz C, Schlagenhauf P, Rath B, et al. A prospective observational safety study on MF59 adjuvanted cell culture‐derived vaccine, Celtura during the A/H1N1 (2009) influenza pandemic. Vaccine 2012;30(45):6436‐43. - PubMed
Rimmelzwaan 2000 {published data only}
-
- Rimmelzwaan GF, Nieuwkoop N, Brandenburg A, Sutter G, Beyer WE, Maher D, et al. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine 2000;19(9‐10):1180‐7. - PubMed
Rocchi 1979c {published data only}
Rowhani‐Rahbar 2012 {published data only}
-
- Rowhani‐Rahbar A, Klein NP, Lewis N, Fireman B, Ray P, Rasgon B, et al. Immunization and Bell's palsy in children: a case‐centered analysis. American Journal of Epidemiology 2012;175(9):878‐85. - PubMed
Ruben 1972 {published data only}
-
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. Journal of Infectious Diseases 1972;125(6):656‐64. - PubMed
Ruben 1973 {published data only}
-
- Ruben FL, Akers LW, Stanley ED, Jackson GG. Protection with split and whole virus vaccines against influenza. Archives of Internal Medicine 1973;132(4):568‐71. - PubMed
Safranek 1991 {published data only}
-
- Safranek TJ, Lawrence DN, Kurland LT, Culver DH, Wiederholt WC, Hayner NS, et al. Reassessment of the association between Guillain‐Barre syndrome and receipt of swine influenza vaccine in 1976‐1977: results of a two‐state study. Expert Neurology Group. American Journal of Epidemiology 1991;133(9):940‐51. - PubMed
Sarateanu 1980 {published data only}
-
- Sarateanu DE, Ehrengut W, Pressler K, Peukert M, Schenk KD. Serological response to whole, split and subunit influenza vaccines of persons with and without immunological experience towards influenza A/U.S.S.R. 90/77 virus. Comparative Immunology, Microbiology and Infectious Diseases 1980;3(1‐2):225‐36. - PubMed
Scheifele 2013 {published data only}
-
- Scheifele DW, Dionne M, Ward BJ, Cooper C, Vanderkooi OG, Li Y, et al. Safety and immunogenicity of 2010‐2011 A/H1N1pdm09‐containing trivalent inactivated influenza vaccine in adults previously given AS03‐adjuvanted H1N1 2009 pandemic vaccine: results of a randomised trial. Human Vaccines and Immunotherapy 2013;9(1):136‐43. - PMC - PubMed
Schonberger 1981 {published data only}
-
- Schonberger LB, Hurwitz ES, Katona P, Holman RC, Bregman DJ. Guillain‐Barré syndrome: its epidemiology and associations with influenza vaccination. Annals of Neurology 1981;9(Suppl):31‐8. - PubMed
Schwartz 1996 {published data only}
-
- Schwartz K. Influenza vaccine for healthy adults. Journal of Family Practice 1996;42(4):351‐2. - PubMed
Simpson 2012 {published data only}
-
- Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: a retrospective observational cohort study. Lancet Infectious Diseases 2012;12(9):696‐702. - PubMed
Sipilä 2015 {published data only}
-
- Sipilä JO, Soilu‐Hanninen M. The incidence and triggers of adult‐onset Guillain‐Barre syndrome in southwestern Finland 2004‐2013. European Journal of Neurology 2015;22(2):292‐8. - PubMed
Skowronski 2002 {published data only}
Skowronski 2003 {published data only}
-
- Skowronski DM, Serres G, Scheifele D, Russell ML, Warrington R, Davies HD, et al. Randomized, double‐blind, placebo‐controlled trial to assess the rate of recurrence of oculorespiratory syndrome following influenza vaccination among persons previously affected. Clinical Infectious Diseases 2003;37(8):1059‐66. - PubMed
Smith 1977a {published data only}
Smith 1977b {published data only}
-
- Smith CD, Leighton HA, Shiromoto RS. Antigenicity and reactivity of influenza A/New Jersey/8/76 virus vaccines in military volunteers at Fort Ord, California. Journal of Infectious Diseases 1977;136(Suppl):460‐5. - PubMed
Song 2011 {published data only}
Souayah 2011 {published data only}
-
- Souayah N, Michas‐Martin PA, Nasar A, Krivitskaya N, Yacoub HA, Khan H, et al. Guillain‐Barré syndrome after Gardasil vaccination: data from Vaccine Adverse Event Reporting System 2006‐2009. Vaccine 2011;29(5):886‐9. - PubMed
Spencer 1975 {published data only}
-
- Spencer MJ, Cherry JD, Powell KR, Sumaya CV, Garakian AJ. Clinical trials with Alice strain, live attenuated, serum inhibitor‐resistant intranasal influenza A vaccine. Journal of Infectious Diseases 1975;132(4):415‐20. - PubMed
Spencer 1979 {published data only}
-
- Spencer MJ, Cherry JD, Powell KR, Sumaya CV. A clinical trial with Alice/R‐75 strain, live attenuated serum inhibitor‐resistant intranasal bivalent influenza A/B vaccine. Medical Microbiology and Immunology 1979;167(1):1‐9. - PubMed
Steinhoff 2012 {published data only}
Sumaya 1979 {published data only}
-
- Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. Journal of Infectious Diseases 1979;140(2):141‐6. - PubMed
Talaat 2010 {published data only}
-
- Talaat KR, Greenberg ME, Lai MH, Hartel GF, Wichems CH, Rockman S, et al. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. Journal of Infectious Diseases 2010;202(9):1327‐37. - PubMed
Tavares 2011 {published data only}
-
- Tavares F, Nazareth I, Monegal JS, Kolte I, Verstraeten T, Bauchau V. Pregnancy and safety outcomes in women vaccinated with an AS03‐adjuvanted split virion H1N1 (2009) pandemic influenza vaccine during pregnancy: a prospective cohort study. Vaccine 2011;29(37):6358‐65. - PubMed
Taylor 1969 {published data only}
-
- Taylor PJ, Miller CL, Pollock TM, Perkins FT, Westwood MA. Antibody response and reactions to aqueous influenza vaccine, simple emulsion vaccine and multiple emulsion vaccine. A report to the Medical Research Council Committee on influenza and other respiratory virus vaccines. Journal of Hygiene 1969;67(3):485‐90. - PMC - PubMed
Taylor 2012 {published data only}
-
- Taylor DN, Treanor JJ, Sheldon EA, Johnson C, Umlauf S, Song L, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza‐flagellin fusion vaccine with improved safety and immune response. Vaccine 2012;30(39):5761‐9. - PubMed
Thompson 2014 {published data only}
-
- Thompson MG, Li DK, Shifflett P, Sokolow LZ, Ferber JR, Kurosky S, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population‐based case‐control study during the 2010‐2011 and 2011‐2012 influenza seasons. Clinical Infectious Diseases 2014;58(4):449‐57. - PubMed
Tokars 2012 {published data only}
-
- Tokars JI, Lewis P, Destefano F, Wise M, Viray M, Morgan O, et al. The risk of Guillain‐Barré syndrome associated with influenza a (H1N1) 2009 monovalent vaccine and 2009‐2010 seasonal influenza vaccines: results from self‐controlled analyses. Pharmacoepidemiology and Drug Safety 2012;21(5):546‐52. - PubMed
Treanor 2001 {published data only}
-
- Treanor JJ, Wilkinson BE, Masseoud F, Hu‐Primmer J, Battaglia R, O'Brien D, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 2001;19(13‐4):1732‐7. - PubMed
Treanor 2002 {published data only}
-
- Treanor J, Keitel W, Belshe R, Campbell J, Schiff G, Zangwill K, et al. Evaluation of a single dose of half strength inactivated influenza vaccine in healthy adults. Vaccine 2002;20(7‐8):1099‐105. - PubMed
Treanor 2012 {published data only}
Tsai 2010 {published data only}
-
- Tsai T, Kyaw MH, Novicki D, Nacci P, Rai S, Clemens R. Exposure to MF59‐adjuvanted influenza vaccines during pregnancy ‐ a retrospective analysis. Vaccine 2010;28(7):1877‐80. - PubMed
Tsatsaris 2011 {published data only}
-
- Tsatsaris V, Capitant C, Schmitz T, Chazallon C, Bulifon S, Riethmuller D, et al. Maternal immune response and neonatal seroprotection from a single dose of a monovalent nonadjuvanted 2009 influenza A(H1N1) vaccine: a single‐group trial. Annals of Internal Medicine 2011;155(11):733‐41. - PubMed
Tyrrell 1970 {published data only}
Vesikari 2012 {published data only}
-
- Vesikari T, Forstén A, Herbinger KH, Cioppa GD, Beygo J, Borkowski A, et al. Safety and immunogenicity of an MF59‐adjuvanted A/H5N1 pre‐pandemic influenza vaccine in adults and the elderly. Vaccine 2012;30(7):1388‐96. - PubMed
Warren‐Gash 2013 {published data only}
Warshauer 1976 {published data only}
-
- Warshauer DM, Minor TE, Inhorn SL, Reed CE, Dick EC. Use of an inhibitor‐resistant live attenuated influenza vaccine in normal and asthmatic adults. 14th Congress of the International Association of Biological Standardization, Douglas, Isle of Man 1975. Developments in Biological Standardization 1975;33:184‐90. - PubMed
Wilde 1999 {published data only}
-
- Wilde JA, McMillan JA, Serwint J, Butta J, O'Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals. JAMA 1999;281(10):908‐13. - PubMed
Williams 1973 {published data only}
Williams 2011 {published data only}
Wise 2012 {published data only}
Wood 1999 {published data only}
-
- Wood SC, Alexseiv A, Nguyen VH. Effectiveness and economical impact of vaccination against influenza among a working population in Moscow. Vaccine 1999;17(Suppl 3):81‐7. - PubMed
Wood 2000 {published data only}
-
- Wood SC, Nguyen VH, Schmidt C. Economic evaluations of influenza vaccination in healthy working‐age adults. Employer and society perspective. Pharmacoeconomics 2000;18(2):173‐83. - PubMed
Xu 2012 {published data only}
-
- Xu R, Luo Y, Chambers C. Assessing the effect of vaccine on spontaneous abortion using time‐dependent covariates Cox models. Pharmacoepidemiology and Drug Safety 2012;21(8):844‐50. - PubMed
Yang 2012 {published data only}
Yeager 1999 {published data only}
-
- Yeager DP, Toy EC, Baker B 3rd. Influenza vaccination in pregnancy. American Journal of Perinatology 1999;16(6):283‐6. - PubMed
Yih 2012 {published data only}
-
- Yih WK, Lee GM, Lieu TA, Ball R, Kulldorff M, Rett M, et al. Surveillance for adverse events following receipt of pandemic 2009 H1N1 vaccine in the Post‐Licensure Rapid Immunization Safety Monitoring (PRISM) System, 2009‐2010. American Journal of Epidemiology 2012;175(11):1120‐8. - PubMed
Zaman 2008 {published data only}
-
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. New England Journal of Medicine 2008;359(15):1555‐64. - PubMed
Additional references
ACIP 2006
-
- Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Recommendations and Reports: Morbidity and Mortality Weekly Report 2006;55:1‐41. - PubMed
ACIP 2010
-
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. Morbidity and Mortality Weekly Report 2010;59(RR‐8):1‐62. - PubMed
ACIP 2015
AIH 2013
-
- Australian Technical Advisory Group on Immunisation (ATAGI). The Australian Immunisation Handbook. 10th Edition. Australian Government: National Health and Medical Research Council, 2013.
Atkins 2004
Demicheli 2014
DiazGranados 2012
-
- DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non‐elderly adults: a systematic review with meta‐analyses of controlled trials. Vaccine 2012;31(1):49‐57. - PubMed
DoH 2015
-
- Department of Health, UK. Influenza. Immunisation Against Infectious Diseases: The Green Book. Vol. 19, Department of Health, UK, 2015:18‐9.
EMA 2014
-
- Committee for Medicinal Products for Human Use. Guideline on influenza vaccines. Non‐clinical and clinical module. European Medicines Agency, 2014, EMA/CHMP/VWP/457259/2014. [web:: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
Farez 2011
-
- Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta‐analysis. Journal of Neurology 2011;258(7):1197‐206. - PubMed
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grohskopf 2016
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jefferson 2009a
-
- Jefferson TO. Mistaken identity: seasonal influenza versus influenza‐like illness. Clinical Evidence 2009;329:1‐4.
Jefferson 2009b
Jefferson 2012
Jones 2016
-
- Jones M, Fowler R. Immortal time bias in observational studies of time to event outcomes. Journal of Critical Care 2016;36:195‐9. - PubMed
Kissling 2009a
-
- Kissling E, Moren A, Valenciano M. Protocol for case‐control studies to measure pandemic and seasonal influenza vaccine effectiveness in the European Union and European Economic Area Member States. ECDC Technical Document. Paris: European Centre for Disease Prevention and Control, 2009. [web: http://ecdc.europa.eu/en/publications/Publications/0907_TED_Influenza_AH... last accessed on May 25th, 2017]
Kissling 2009b
-
- Kissling E, Moren A, Valenciano M. Protocol for cohort database studies to measure pandemic and seasonal influenza vaccine effectiveness in the European Union and European Economic Area Member States. ECDC Technical Document. Paris: European Centre for Disease Prevention and Control, 2009. [web: http://ecdc.europa.eu/en/publications/Publications/0907_TER_Influenza_AH... Last accessed on May 25th, 2017]
Moher 2009
NACI 2014
-
- National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS) National Advisory Commitee on Immunization: statement on seasonal influenza vaccine for 2014‐2015. Canada Communicable Diseases Report, 2014. http:/www.phac‐aspc.gcca/naci‐ccni/assets/pdf/flu‐grippe‐eng.pdf (accessed September 3, 2015).
Osterholm 2012
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta‐analysis. Lancet Infectious Diseases 2012;12:36‐44. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Skowronski 2009
-
- Skowronski DM, Serres G. Is routine immunisation warranted in early pregnancy?. Vaccine 2009;27:4754‐70. - PubMed
STIKO 2010
-
- Ständigen Impfkommission (STIKO) am Robert Koch‐Institut. Recommendations update for immunisation against influenza [Änderung der Empfehlungen zur Impfung gegen Influenza]. Epidemiologisches Bulletin 2010;31:299‐308.
Toback 2012
-
- Toback SL, Levin MJ, Block SL, Belshe RB, Ambrose CS, Falloon J. Quadrivalent Ann Arbor strain live‐attenuated influenza vaccine. Expert Reviews Vaccines 2012;11(11):1293‐303. - PubMed
Treanor 2016
Wijnans 2016
References to other published versions of this review
Demicheli 1998
-
- Demicheli V, Jefferson T. Influenza vaccines in healthy adults. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001269] - DOI
Demicheli 1999
-
- Demicheli V, Rivetti D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001269] - DOI
Demicheli 2001
Demicheli 2004
Jefferson 2007
Jefferson 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

