Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI)
- PMID: 29388198
- PMCID: PMC6491064
- DOI: 10.1002/14651858.CD012693.pub2
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI)
Update in
-
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI).Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD012693. doi: 10.1002/14651858.CD012693.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174816 Free PMC article. Review.
Abstract
Background: During a cycle of in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI), women receive daily doses of gonadotropin follicle-stimulating hormone (FSH) to induce multifollicular development in the ovaries. Generally, the dose of FSH is associated with the number of eggs retrieved. A normal response to stimulation is often considered desirable, for example the retrieval of 5 to 15 oocytes. Both poor and hyper-response are associated with increased chance of cycle cancellation. Hyper-response is also associated with increased risk of ovarian hyperstimulation syndrome (OHSS). Clinicians often individualise the FSH dose using patient characteristics predictive of ovarian response such as age. More recently, clinicians have begun using ovarian reserve tests (ORTs) to predict ovarian response based on the measurement of various biomarkers, including basal FSH (bFSH), antral follicle count (AFC), and anti-Müllerian hormone (AMH). It is unclear whether individualising FSH dose based on these markers improves clinical outcomes.
Objectives: To assess the effects of individualised gonadotropin dose selection using markers of ovarian reserve in women undergoing IVF/ICSI.
Search methods: We searched the Cochrane Gynaecology and Fertility Group Specialised Register, Cochrane Central Register of Studies Online, MEDLINE, Embase, CINAHL, LILACS, DARE, ISI Web of Knowledge, ClinicalTrials.gov, and the World Health Organisation International Trials Registry Platform search portal from inception to 27th July 2017. We checked the reference lists of relevant reviews and included studies.
Selection criteria: We included trials that compared different doses of FSH in women with a defined ORT profile (i.e. predicted low, normal or high responders based on AMH, AFC, and/or bFSH) and trials that compared an individualised dosing strategy (based on at least one ORT measure) versus uniform dosing or a different individualised dosing algorithm.
Data collection and analysis: We used standard methodological procedures recommended by Cochrane. Primary outcomes were live birth/ongoing pregnancy and severe OHSS. Secondary outcomes included clinical pregnancy, moderate or severe OHSS, multiple pregnancy, oocyte yield, cycle cancellations, and total dose and duration of FSH administration.
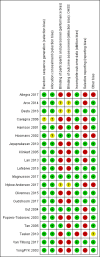
Main results: We included 20 trials (N = 6088); however, we treated those trials with multiple comparisons as separate trials for the purpose of this review. Meta-analysis was limited due to clinical heterogeneity. Evidence quality ranged from very low to moderate. The main limitations were imprecision and risk of bias associated with lack of blinding.Direct dose comparisons in women according to predicted responseAll evidence was low or very low quality.Due to differences in dose comparisons, caution is warranted in interpreting the findings of five small trials assessing predicted low responders. The effect estimates were very imprecise, and increased FSH dosing may or may not have an impact on rates of live birth/ongoing pregnancy, OHSS, and clinical pregnancy.Similarly, in predicted normal responders (nine studies, three comparisons), higher doses may or may not impact the probability of live birth/ongoing pregnancy (e.g. 200 versus 100 international units: OR 0.88, 95% CI 0.57 to 1.36; N = 522; 2 studies; I2 = 0%) or clinical pregnancy. Results were imprecise, and a small benefit or harm remains possible. There were too few events for the outcome of OHSS to enable any inferences.In predicted high responders, lower doses may or may not have an impact on rates of live birth/ongoing pregnancy (OR 0.98, 95% CI 0.66 to 1.46; N = 521; 1 study), OHSS, and clinical pregnancy. However, lower doses probably reduce the likelihood of moderate or severe OHSS (Peto OR 2.31, 95% CI 0.80 to 6.67; N = 521; 1 study).ORT-algorithm studiesFour trials compared an ORT-based algorithm to a non-ORT control group. Rates of live birth/ongoing pregnancy and clinical pregnancy did not appear to differ by more than a few percentage points (respectively: OR 1.04, 95% CI 0.88 to 1.23; N = 2823, 4 studies; I2 = 34%; OR 0.96, 95% CI 0.82 to 1.13, 4 studies, I2=0%, moderate-quality evidence). However, ORT algorithms probably reduce the likelihood of moderate or severe OHSS (Peto OR 0.58, 95% CI 0.34 to 1.00; N = 2823; 4 studies; I2 = 0%, low quality evidence). There was insufficient evidence to determine whether the groups differed in rates of severe OHSS (Peto OR 0.54, 95% CI 0.14 to 1.99; N = 1494; 3 studies; I2 = 0%, low quality evidence). Our findings suggest that if the chance of live birth with a standard dose is 26%, the chance with ORT-based dosing would be between 24% and 30%. If the chance of moderate or severe OHSS with a standard dose is 2.5%, the chance with ORT-based dosing would be between 0.8% and 2.5%. These results should be treated cautiously due to heterogeneity in the study designs.
Authors' conclusions: We did not find that tailoring the FSH dose in any particular ORT population (low, normal, high ORT), influenced rates of live birth/ongoing pregnancy but we could not rule out differences, due to sample size limitations. In predicted high responders, lower doses of FSH seemed to reduce the overall incidence of moderate and severe OHSS. Moderate-quality evidence suggests that ORT-based individualisation produces similar live birth/ongoing pregnancy rates to a policy of giving all women 150 IU. However, in all cases the confidence intervals are consistent with an increase or decrease in the rate of around five percentage points with ORT-based dosing (e.g. from 25% to 20% or 30%). Although small, a difference of this magnitude could be important to many women. Further, ORT algorithms reduced the incidence of OHSS compared to standard dosing of 150 IU, probably by facilitating dose reductions in women with a predicted high response. However, the size of the effect is unclear. The included studies were heterogeneous in design, which limited the interpretation of pooled estimates, and many of the included studies had a serious risk of bias.Current evidence does not provide a clear justification for adjusting the standard dose of 150 IU in the case of poor or normal responders, especially as increased dose is generally associated with greater total FSH dose and therefore greater cost. However, a decreased dose in predicted high responders may reduce OHSS.
Conflict of interest statement
Sarah Lensen does not have any conflicts of interest to declare.
Jack Wilkinson was funded by a Doctoral Research Fellowship supported by the National Institute for Health Research while completing part of this work (DRF‐2014‐07‐050). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the National Institute for Health Research or the Department of Health. JW also declares that publishing research is beneficial to his career.
Jori Leijdekkers received an unrestricted personal grant from Merck B.V.
Antonio La Marca received research funding to his institution from companies producing gonadotrophins (Merck Serono, Ferring, IBSA, MSD). He did not receive direct payment for consultancies or conference speaking in the last 3 years from any of these companies. Antonio La Marca is an investigator on a trial included in this review (Allegra 2017). To manage this potential conflict, review authors did not extract data or assess risk of bias for the studies they have been involved with.
Ben Mol has received consultancy fees from ObsEva, Guerbet and Merck; payment for review preparation from European Journal of Obstetrics and Gynecology and Reproductive Biology; and travel/accommodation/meeting expenses for various non‐commercial scientific meetings.
Jane Marjoribanks does not have any conflicts of interest to declare.
Helen Torrance has received travel grants from from Merck B.V.
Frank Broekmans has not consulted for any related companies in the last 3 years.
Frank Broekmans, Ben Mol and Helen Torrance are investigators in the OPTIMIST trial, which is included in this review (Oudshoorn 2017; Van Tilborg 2017). Frank Broekmans is an investigator on two other trials included in this review (Klinkert 2005; Olivennes 2015). To manage this potential conflict, review authors did not extract data or assess risk of bias for the studies they have been involved with.
Figures




















































































































































References
References to studies included in this review
Allegra 2017 {published and unpublished data}
-
- Allegra A, Marino A, Volpes A, Coffaro F, Scaglione P, Gullo A, et al. A randomized controlled trial investigating the use of a predictive nomogram for the selection of the FSH starting dose in IVF/ICSI cycles. Reproductive Biomedicine Online 2017;34(4):429‐38. - PubMed
Arce 2014 {published data only}
-
- Arce JC, Andersen AN, Fernandez‐Sanchez M, Visnova H, Bosch E, Garcia‐Velasco JA, et al. Ovarian response to recombinant human follicle‐stimulating hormone: a randomized, antimullerian hormone‐stratified, dose response trial in women undergoing in vitro fertilization/ intracytoplasmic sperm injection. Fertility and Sterility 2014;102(6):1633‐40. - PubMed
Bastu 2016 {published and unpublished data}
-
- Bastu E, Buyru F, Ozsurmeli M, Demiral I, Dogan M, Yeh J. A randomized, single‐blind, prospective trial comparing three different gonadotropin doses with or without addition of letrozole during ovulation stimulation in patients with poor ovarian response. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;203:30‐4. - PubMed
Cavagna 2006 {published data only}
-
- Cavagna M, Dzik A, Freitas GC, Soares JB, Pawn K, Sales ALM, et al. Comparison of 150 IU and 225 IU of follitropin‐beta in a fixed‐dose regimen for ovarian stimulation using a depot formulation of GnRH agonist: a prospective randomized clinical trial. Jornal Brasileiro de Reproducao Assistida 2006;10(2):21‐4.
Harrison 2001 {published data only}
-
- Harrison RF, Jacob S, Spillane H, Mallon E, Hennelly B. A prospective randomized clinical trial of differing starter doses of recombinant follicle‐stimulating hormone (follitropin‐β) for first time in vitro fertilization and intracytoplasmic sperm injection treatment cycles. Fertility and Sterility 2001;75(1):23‐31. - PubMed
Hoomans 2002 {published data only}
-
- Hoomans EHM, Mulder BB, on behalf of the Asian Puregon Study Group. A group‐comparative, randomized, double‐blind comparison of the efficacy and efficiency of two fixed daily dose regimens (100‐ and 200‐IU) of recombinant follicle stimulating hormone (rFSH, Puregon) in Asian women undergoing ovarian stimulation for IVF/ICSI. Journal of Assisted Reproduction and Genetics 2002;19(10):470‐6. - PMC - PubMed
Jayaprakasan 2010 {published and unpublished data}
-
- Jayaprakasan K, Hopkisson J, Campbell B, Johnson I, Thornton J, Raine‐Fenning N. A randomised controlled trial of 300 versus225 IU recombinant FSH for ovarian stimulation in predicted normal responders by antral follicle count. British Journal of Obstetrics and Gynaecology 2010;117:853‐62. - PubMed
Klinkert 2005 {published and unpublished data}
-
- Klinkert ER, Broekmans FJM, Looman CWN, Habbema JDF, Velde ER. Expected poor responders on the basis of an antral follicle count do not benefit from a higher starting dose of gonadotrophins in IVF treatment: a randomized controlled trial. Human Reproduction 2005;20(3):611‐5. - PubMed
Lan 2013 {published and unpublished data}
-
- Lan VTN, Linh NK, Tuong HM, Wong PC, Howles CM. Anti‐Mullerian hormone versus antral follicle count for defining the starting dose of FSH. Reproductive BioMedicine Online 2013;27(4):390‐9. - PubMed
Lefebrve 2015 {published and unpublished data}
-
- Lefebvre J, Antaki R, Kadoch IJ, Dean NL, Sylvestre C, Bissonnette F, et al. 450 IU versus 600 IU gonadotropin for controlled ovarian stimulation in poor responders: a randomized controlled trial. Fertility and Sterility 2015;104(6):1419‐25. - PubMed
Magnusson 2017 {published and unpublished data}
-
- Magnusson Å, Nilsson L, Oleröd G, Thurin‐Kjellberg1 A, Bergh C. The addition of anti‐Müllerian hormone in an algorithm for individualized hormone dosage did not improve the prediction of ovarian response—a randomized, controlled trial. Human Reproduction 2017;32(4):811‐9. - PubMed
Nyboe Andersen 2017 {published and unpublished data}
-
- Nyboe Andersen A, Nelson SM, Fauser BCJM, García‐Velasco JA, Klein BM, Arce JC, ESTHER‐1 study group. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor‐blinded, phase3 noninferiority trial. Fertility and Sterility 2017;107(2):387‐96. - PubMed
Olivennes 2015 {published data only (unpublished sought but not used)}
-
- Olivennes F, Trew G, Borini A, Broekmans F, Arriagada P, Warne DW, Howles CM. Randomized, controlled, open‐label, noninferiority study of the CONSORT algorithm for individualized dosing of follitropin alfa. Reproductive BioMedicine Online 2015;30:248‐57. - PubMed
Oudshoorn 2017 {published data only}
-
- Oudshoorn SC, Tilborg TC, Eijkemans MJC, Oosterhuis GJE, Friederich J, Hooff HA, et al. Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 2: the predicted hyper responder. Human Reproduction 2017;32(12):2506‐14. - PubMed
Out 2004 {published data only}
-
- Out HJ, Rutherford A, Fleming R, Tay CCK, Trew G, Ledger W, et al. A randomized, double‐blind, multicentre clinical trial comparing starting doses of 150 and 200 IU of recombinant FSH in women treated with the GnRH antagonist ganirelix for assisted reproduction. Human Reproduction 2004;19(1):90‐5. - PubMed
Popovic‐Todorovic 2003 {published and unpublished data}
-
- Popovic‐Todorovic B, Loft A, Bredkjñer HE, Bangsbùll S, Nielsen IK, Nyboe Andersen A. A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a 'standard' dose of 150 IU/day in 'standard' patients undergoing IVF/ICSI treatment. Human Reproduction 2003;18(11):2275‐82. - PubMed
Tan 2005 {published data only}
-
- Tan SL, Child TJ, Cheung AP, Fluker MR, Yuzpe A, Casper R, et al. A randomized, double‐blind, multicenter study comparing a starting dose of 100 IU or 200 IU of recombinant follicle stimulating hormone (Puregon R) in women undergoing controlled ovarian hyperstimulation for IVF treatment. Journal of Assisted Reproduction and Genetics 2005;22(2):81‐8. - PMC - PubMed
Tasker 2010 {published and unpublished data}
-
- Tasker F, Hamoda H, Wilner H, Grace J, Khalaf Y. A randomised controlled trial comparing cycle outcome of individualised versus standard dosage of recombinant FSH with IVF/ICSI treatment. Human Reproduction 2010;25(Suppl 1):i170‐210.
Van Tilborg 2017 {published data only}
-
- Tilborg TC, Torrance HL, Oudshoorn SC, Eijkemans MJC, Koks CAM, Verhoeve HRV, et al. Individualized versus standard FSH dosing in women starting IVF/ICSI: anRCT. Part 1: the predicted poor responder. Human Reproduction 2017;32(12):2496‐2505. - PubMed
YongPYK 2003 {published data only}
-
- Yong PYK, Brett S, Baird DT, Thong KJ. A prospective randomized clinical trial comparing 150 IU and 225 IU of recombinant follicle‐stimulating hormone(Gonal‐F*) in a fixed‐dose regimen for controlled ovarian stimulation in in vitro fertilization treatment. Fertility and Sterility 2003;79(2):308‐15. - PubMed
References to studies excluded from this review
Berkkanoglu 2010 {published data only}
-
- Berkkanoglu M, Ozgur K. What is the optimum maximal gonadotropin dosage used in microdose flare‐up cycles in poor responders?. Fertility and Sterility 2010;94(2):662‐5. - PubMed
Camier 1999 {published data only}
-
- Camier B. A multicentre, prospective, randomised study to compare low dose protocol versus conventional administration of Rh‐FSH (GONAL‐F®) in normo‐responders undergoing ART. Congress of controversies in Obstestrics, Gynaecology and Infertility 1999;1:7.
Cavagna 2009 {published data only}
-
- Cavagna M, Dzik A, Freitas GC, Soares JB, Donadio N, Cavagna F, et al. A prospective and randomized trial comparing 225IU and 300 IU follitropin‐alpha; in a fixed‐dose regimen for controlled ovarian stimulation. Jornal Brasileiro de Reproducao Assistida 2009;24(2):66‐70.
DeJong 2000 {published data only}
-
- Jong D, Macklon NS, Fauser BCJM. A pilot study involving minimal ovarian stimulation for in vitro fertilization: extending the "follicle‐stimulating hormone window" combined with the gonadotropin‐releasing hormone antagonist cetrorelix. Fertility and Sterility 2000;73(5):1051‐4. - PubMed
Fluker 2000 {published data only}
-
- Fluker MR, Copeland JE, Yuzpe AA. A prospective randomized clinical trial of four fixed starting doses of recombinant FSH in women undergoing ovarian stimulation for IVF. Human Reproduction 2000;15:26.
Latin‐American Puregon IVF Study Group 2001 {published data only}
-
- Latin‐American Puregon IVF Study Group. A double‐blind clinical trial comparing a fixed daily dose of 150 and 250 IU of recombinant follicle‐stimulating hormone in women undergoing in vitro fertilization. Fertility and Sterility 2001;76(5):950‐6. - PubMed
NCT02915900 {unpublished data only}
-
- NCT02915900. Optimising FSH dosage during in vitro fertilization (IVF) [Method for Determining Optimal FSH Dosage During in Vitro Fertilization (IVF)]. clinicaltrials.gov/ct2/show/NCT02915900 First received 27 September 2016.
Out 1999 {published data only}
-
- Out HJ, Lindenberg S, Mikkelsen AL, Eldar‐Geva T, Healy DL, Leader A, et al. A prospective, randomized, double‐blind clinical trial to study the efficacy and efficiency of a fixed dose of recombinant follicle stimulating hormone (Puregon) in women undergoing ovarian stimulation. Human Reproduction 1999;14(3):622‐7. - PubMed
Out 2000 {published data only}
-
- Out HJ, Braat DDM, Lintsen BME, Gurgan T, Bukulmez O, Gökmen O, et al. Increasing the daily dose of recombinant follicle stimulating hormone (Puregon) does not compensate for the age‐related decline in retrievable oocytes are ovarian stimulation. 2000 Human Reproduction;15(1):29‐35. - PubMed
Out 2001 {published data only}
-
- Out HJ, David I, Ron‐El R, Friedler S, Shalev E, Geslevich J, et al. A randomized, double‐blind clinical trial using fixed daily doses of 100 IU or 200 IU of recombinant FSH in ICSI cycles. Human Reproduction 2001;16(6):1104‐9. - PubMed
Pruksananonda 2004 {published data only}
-
- Pruksananonda K, Suwajanakorn S, Sereepapong W, Virutamasen P. Comparison of two different fixed doses of follitropin‐β in controlled ovarian hyperstimulation: a prospective randomized, double blind clinical trial. Journal of the Medical Association of Thailand 2004;87(10):1151‐5. - PubMed
Simberg 2000 {published data only}
-
- Simberg N, Rova K, Gudmundsson J, Lundkvist Ö, Lundqvist M, Bekuretsion M, et al. A randomized study comparing Puregon 100 IU or 150 IU as starting dose in IVF treatment. Human Reproduction 2000;Abstract book 1:125.
Tsagareishvili 2005 {published data only}
-
- Tsagareishvili G, Khonelidze N, Lazarev A. Results of application of high daily doses of recombinant follicle stimulating hormone in different age groups of women in IVF program. Georgian Medical News. 2005; Vol. 6:11. - PubMed
Wikland 2001 {published data only}
-
- Wikland M, Bergh C, Borg K, Hillensjö T, Howles CM, Knutsson A, et al. A prospective, randomized comparison of two starting doses of recombinant FSH in combination with cetrorelix in women undergoing ovarian stimulation for IVF/ICSI. Human Reproduction 2001;16(8):1676‐81. - PubMed
References to studies awaiting assessment
NCT02309671 {published data only}
-
- NCT02309671. A Dose‐response Trial Using FE 999049 in Japanese Women Undergoing in Vitro Fertilisation (IVF) / Intracytoplasmic Sperm Injection (ICSI) Treatment. https://clinicaltrials.gov/ct2/show/NCT02309671 First received 5th December 2014.
References to ongoing studies
CTRI/2016/10/007367 {unpublished data only}
-
- CTRI/2016/10/007367. Phase III Study to Evaluate the Efficacy and Safety of Recombinant Human FSH of Cadila Healthcare Limited, India as compared to Gonal‐F Administered Subcutaneously in Female Patients Undergoing Assisted Reproductive Technology. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=16431&EncHid... First received 14th October 2016.
EUCT2012‐004969‐40 {unpublished data only}
-
- EUCT2012‐004969‐40. An AMH based individualised controlled ovarian stimulation regiment using Corifollitrophin Alphs or graded doses of rFSH versus a standard protocol. A randomised controlled trial. www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐004969‐40/DK First received 23rd November 2012.
NCT01794208 {unpublished data only}
-
- NCT01794208. Efficacy and Safety of FSH‐GEX™ in Comparison With 150 IU Gonal‐f®. https://clinicaltrials.gov/ct2/show/NCT01794208 First received 18th February 2013.
NCT02430740 {unpublished data only}
-
- NCT02430740. Tailored Ovarian Stimulation Based on BMI, AMH, AFC. https://clinicaltrials.gov/ct2/show/NCT02430740 First received 30th April 2015.
NCT02739269 {published data only}
-
- NCT02739269. Antimullerian Hormone Versus Antral Follicle Count for Determination of Gonadotrophin Dosing in IVF. https://clinicaltrials.gov/ct2/show/NCT02739269 First received 15th April 2016.
Singh 2015 {published data only}
-
- Singh M, Singh R, Jindal A, Jindal PC. A prospective randomised controlled trial (RCT) on the role of AMH tailored stimulation protocols (Agonist or Antagonist), in improving IVF outcome in previous failed cycles. Human Reproduction. 2015; Vol. 30:i419‐20, P‐699.
Additional references
Alam 2017 [pers comm]
-
- Alam V. Query on trial: CONSORT vs 150 for FSH dosing. Lensen S 31st May 2017.
Aljawoan 2012
Andersen 2006
-
- Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor‐blind controlled trial. Human Reproduction 2006;21(12):3217‐27. - PubMed
Arce 2013
-
- Arce JC, Marca A, Mirner Klein B, Nyboe Andersen A, Fleming R. Antimüllerian hormone in gonadotropin releasing‐hormone antagonist cycles: prediction of ovarian response and cumulative treatment outcome in good‐prognosis patients. Fertility and sterility 2013;99(6):1644‐53. - PubMed
ASRM 2016
-
- Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertility and Sterility 2016;106:1634‐47. - PubMed
Bastu 2017 [pers comm]
-
- Bastu E. Query on trial: 450 vs 300 IU FSH. Eur J Obstet Gynecol Reprod Biol 2016. Lensen S 15 May 2017.
Berkkanoglu 2017 [pers comm]
-
- Berkkanoglu M. Query on trial: 600 vs 450 vs 300 IU FSH. Fertility and sterility 2010. Lensen S 4th May 2017.
Bourgain 2003
-
- Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Human Reproduction Update 2003;9:515‐22. - PubMed
Broekmans 2006
-
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Human Reproduction Update 2006;12:685‐718. - PubMed
Broekmans 2010
-
- Broekmans FJ, Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertility and Sterility 2010;94(3):1044‐51. - PubMed
Broekmans 2017 [pers comm]
-
- Broekmans F. Query on trial: Klinkert 2005. Lensen S 20 May 2017.
Broer 2013a
-
- Broer SL, Dólleman M, Disseldorp J, Broeze KA, Opmeer BC, Bossuyt PM, et al. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta‐analysis. Fertility and Sterility 2013;100(2):420‐9. - PubMed
Broer 2013b
-
- Broer SL, Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, et al. on behalf of the IMPORT study group. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Human Reproduction Update 2013;19(1):26‐36. - PubMed
Broer 2014
-
- Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM. Anti‐Müllerian hormone: ovarian reserve testing and its potential clinical implications. Human Reproduction Update 2014;20(5):688‐701. - PubMed
Calhaz‐Jorge 2016
-
- Calhaz‐Jorge C, Geyter C, Kupka MS, de Mouzon, Erb K, Mocanu E, et al. Assisted reproductive technology in Europe, 2012: results generated from European registers: results generated from European registers by ESHRE. Human Reproduction 2016;31(8):1638‐52. - PubMed
Dyer 2016
-
- Dyer S, Chambers GM, Mouzon J, Nygren KG, Zegers‐Hochschild F, Mansour R, et al. International committee for monitoring assisted reproductive technologies world report: Assistedreproductive technology 2008, 2009 and 2010. Human Reproduction 2016;31(7):1588‐1690. - PubMed
Emsley 2009
-
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Statistical Methods in Medical Research 2009;19(3):237‐70. - PubMed
Fauser 2017
-
- Fauser BCJM. Patient‐tailored ovarian stimulation for in vitro fertilization. Fertility and Sterility 2017;108(4):585‐91. - PubMed
Ferraretti 2011
-
- Ferraretti A, Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Human Reproduction 2011;26(7):1616‐24. - PubMed
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 1 June 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gunby 2010
-
- Gunby J, Bissonnette F, Librach C, Cowan L. Assisted reproductive technologies (ART) in Canada: 2006 results from the Canadian ART Register. Fertility and Sterility 2010;93:2189‐201. - PubMed
Hamoda 2017 [pers comm]
-
- Hamoda H. Query on trial: individualised versus standard dosage of recombinant FSH. Lensen S 27th March 2017.
Harbin Consensus Workshop Group 2014
Harris 2016
-
- Harris K, Fitzgerald O, Paul R C, Macaldowie A, Lee E, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2014. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales, 2016.
Helmgaard 2017 [pers comm]
-
- Helmgaard L. Query on trial: Arce 2017. Lensen S 7 July 2017.
Helmgaard 2017b [pers comm]
-
- Helmgaard L. n/a. Lensen S 7th July 2017.
Higgins 2003
Higgins 2008
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Jayaprakasan 2017 [pers comm]
-
- Jayaprakasan K. Query on trial: 225 v 300 IU FSH. 2010. Lensen S 30 May 2017.
Kawwass 2015
-
- Kawwass JF, Kissin DM, Kulkarni AD, Creanga AA, Session DR, Callaghan WM, et al. Safety of assisted reproductive technology in the United States, 2000‐2011. JAMA 2015;313(1):88. - PubMed
Kolibianakis 2002
-
- Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle‐stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick‐up. Fertility and Sterility 2002;78:1025‐9. - PubMed
Kupka 2014
-
- Kupka MS, Ferraretti AP, Mouzon J, Erb K, D'Hooghe T, Castilla JA, et al. European IVF‐Monitoring Consortium, for the European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Human Reproduction 2014;29(10):2099‐113. - PubMed
La Marca 2012
-
- Marca A, Papeleo E, Grisendi V, Argento C, Giulini S, Volpe A. Development of a nomogram based on markers of ovarian reserve for the individualisation of the follicle‐stimulating hormone starting dose in in vitro fertilisation cycles. BJOG 2012;119(10):1171‐9. - PubMed
La Marca 2014
-
- Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Human Reproduction Update 2014;20(1):124‐40. - PubMed
La Marca 2017
-
- Marca A, Minasi MG, Sighinolfi G, Greco P, Argento C, Grisendi V, et al. Female age, serum antimüllerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertility and Sterility 2017;108(5):777‐83. - PubMed
La Marca 2017 [pers comm]
-
- Marca A. Query on trial: Nomogram for FSH dosing 2017. Lensen S 24 March 2017.
Labarta 2017
Lan 2017 [pers comm]
-
- Lan TN. Query on trial: AMH vs AFC trial for FSH dosing. Lensen S 18 May 2017.
Lefebvre 2017 [pers comm]
-
- Lefebvre J. Query on trial: 450 vs 600 IU FSH. Lefebvre 2015. Lensen S 15th May 2017.
Lekamge 2008
Magnusson 2017 [pers comm]
-
- Magnusson A. Query on trial: Use of AMH in an algorithm for IVF. HR 2017. Lensen S 17th May 2017.
McGowan 1985
-
- McGowan MR, Braithwaite M, Jochle W, Maplecoft RJ. Superovulation of beef heifers with Pergonal (HMG): a dose response trial. Theriogenology 1985;24(2):173‐84. - PubMed
O'Brien 1979
-
- O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35(3):549‐57. - PubMed
Pandian 2010
Popovic‐Todorovic 2017 [pers comm]
-
- Popovic‐Todorovic B. Query on trial 150 vs 100‐250 FSH for IVF. Lensen S 31st May 2017.
Roberts 2005
-
- Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, et al. Follicle‐stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biology of Reproduction 2005;72(1):107‐18. - PubMed
Rustamov 2017
Seifer 2002
-
- Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian‐inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertility and Sterility 2002;77(3):468‐71. - PubMed
Sterrenburg 2011
-
- Sterrenburg MD, Veltman‐Verhulst SM, Eijkemans MJC, Hughes EG, Macklon NS, Broekmans FJ, et al. Clinical outcomes in relation to the daily dose of recombinant follicle stimulating hormone for ovarian stimulation in in vitro fertilization in presumed normal responders younger than 39 years: a meta‐analysis. Human Reproduction Update 2011;17(2):184‐96. - PubMed
Steward 2014
-
- Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertility and Sterility 2014;101(4):967‐73. - PubMed
Sugano 1997
-
- Sugano M, Watanabe S. Use of highly purified porcine FSH preparation for superovulation in Japanese black cattle. Journal of Veterinary Medical Science 1997;59(3):223‐5. - PubMed
Sunkara 2011
-
- Sunkara SK, Rittenberg V, Raine‐Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Human Reproduction 2011;26(7):1768‐74. - PubMed
Tajik 2013
-
- Tajik P, Zwinderman AH, Mol BW, Bossuyt PM. Trial designs for personalizing cancer care: a systematic review and classification. Clinical Cancer Research 2013;19(17):4578‐88. - PubMed
Thoma 2013
Thong 2017 [pers comm]
-
- Thong KJ. Query on trial: 150 v 225 IU FSH. Yong 2003. Lensen S 13th April 2017.
Ting 2009
-
- Ting PY, Wen XY. The influence of FSH on the aneuploidy rate of human in vitro matured oocytes. Fertility and Sterility 2009;92(3):S14.
Toftager 2017
-
- Toftager M, Bogstad J, Lossl K, Praetorius L, Zedeler A, Bryndorf T, et al. Cumulative live birthrates after one ART cycle including all subsequent frozen‐thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH‐antagonist and GnRH‐agonist protocols. Human Reproduction 2017;32(3):556‐67. - PubMed
Torrance 2017 [pers comm]
-
- Torrance H. Query on van Tilborg 2017. Lensen S 12th September 2017.
Trew 2017 [pers comm]
-
- Trew G. Query on study: Out 2004. Lensen S 18th April 2017.
Vail 2003
-
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18:1000‐4. - PubMed
Van Rooij 2002
-
- Rooij IA, Broekmans FJ, Velde ER, Fauser BC, Bancsi LF, Jong FH, et al. Serum anti‐Müllerian hormone levels: a novel measure of ovarian reserve. Human Reproduction 2002;17(12):3065‐71. - PubMed
Van Tilborg 2016
-
- Tilborg TC, Broekmans FJM, Dolleman M, Eijkemans MJC, Mol BW, Laven JSE. Individualized follicle‐stimulating hormone dosing and in vitro fertilization outcome in agonist downregulated cycles: a systematic review. Acta Obstetricia et Gynecologica Scandinavica 2016;95:1333‐44. - PubMed
Visser 2006
-
- Visser JA, Jong FH, Laven JS, Themmen AP. Anti‐Müllerian hormone: a new marker for ovarian function. Reproduction 2006;131(1):1‐9. - PubMed
Wong 2017
References to other published versions of this review
Lensen
-
- Lensen SF, Wilkinson J, Mol BWJ, Marca A, Torrance H, Broekmans FJ. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing IVF/ICSI. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing IVF/ICSI. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD012693] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

