PLCγ1-PKCε-IP3R1 signaling plays an important role in hypoxia-induced calcium response in pulmonary artery smooth muscle cells
- PMID: 29388468
- PMCID: PMC6008133
- DOI: 10.1152/ajplung.00243.2017
PLCγ1-PKCε-IP3R1 signaling plays an important role in hypoxia-induced calcium response in pulmonary artery smooth muscle cells
Abstract
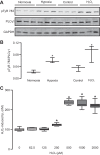
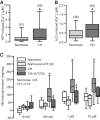
Hypoxia-induced pulmonary vasoconstriction (HPV) is attributed to an increase in intracellular Ca2+ concentration ([Ca2+]i) in pulmonary artery smooth muscle cells (PASMCs). We have reported that phospholipase C-γ1 (PLCγ1) plays a significant role in the hypoxia-induced increase in [Ca2+]i in PASMCs and attendant HPV. In this study, we intended to determine molecular mechanisms for hypoxic Ca2+ and contractile responses in PASMCs. Our data reveal that hypoxic vasoconstriction occurs in pulmonary arteries, but not in mesenteric arteries. Hypoxia caused a large increase in [Ca2+]i in PASMCs, which is diminished by the PLC inhibitor U73122 and not by its inactive analog U73433 . Hypoxia augments PLCγ1-dependent inositol 1,4,5-trisphosphate (IP3) generation. Exogenous ROS, hydrogen peroxide (H2O2), increases PLCγ1 phosphorylation at tyrosine-783 and IP3 production. IP3 receptor-1 (IP3R1) knock-down remarkably diminishes hypoxia- or H2O2-induced increase in [Ca2+]i. Hypoxia or H2O2 increases the activity of IP3Rs, which is significantly reduced in protein kinase C-ε (PKCε) knockout PASMCs. A higher PLCγ1 expression, activity, and basal [Ca2+]i are found in PASMCs, but not in mesenteric artery smooth muscle cells from mice exposed to chronic hypoxia (CH) for 21 days. CH enhances H2O2- and ATP-induced increase in [Ca2+]i in PASMCs and PLC-dependent, norepinephrine-evoked pulmonary vasoconstriction. In conclusion, acute hypoxia uniquely causes ROS-dependent PLCγ1 activation, IP3 production, PKCε activation, IP3R1 opening, Ca2+ release, and contraction in mouse PASMCs; CH enhances PASM PLCγ1 expression, activity, and function, playing an essential role in pulmonary hypertension in mice.
Keywords: calcium; hypoxia; hypoxia-induced pulmonary vasoconstriction; inositol 1,4,5-trisphosphate receptor-1; phospholipase C-γ1; protein kinase C-ε; reactive oxygen species.
Figures







Similar articles
-
Important role of PLC-γ1 in hypoxic increase in intracellular calcium in pulmonary arterial smooth muscle cells.Am J Physiol Lung Cell Mol Physiol. 2013 Feb 1;304(3):L143-51. doi: 10.1152/ajplung.00310.2012. Epub 2012 Nov 30. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23204067 Free PMC article.
-
Mitochondrial ROS-PKCepsilon signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells.Biochem Biophys Res Commun. 2006 Dec 22;351(3):784-90. doi: 10.1016/j.bbrc.2006.10.116. Epub 2006 Oct 30. Biochem Biophys Res Commun. 2006. PMID: 17087917 Free PMC article.
-
Type 2 inositol 1,4,5-trisphosphate receptor inhibits the progression of pulmonary arterial hypertension via calcium signaling and apoptosis.Heart Vessels. 2019 Apr;34(4):724-734. doi: 10.1007/s00380-018-1304-4. Epub 2018 Nov 20. Heart Vessels. 2019. PMID: 30460575
-
ROS-dependent signaling mechanisms for hypoxic Ca(2+) responses in pulmonary artery myocytes.Antioxid Redox Signal. 2010 Mar 1;12(5):611-23. doi: 10.1089/ars.2009.2877. Antioxid Redox Signal. 2010. PMID: 19764882 Free PMC article. Review.
-
Cross Talk Between Mitochondrial Reactive Oxygen Species and Sarcoplasmic Reticulum Calcium in Pulmonary Arterial Smooth Muscle Cells.Adv Exp Med Biol. 2017;967:289-298. doi: 10.1007/978-3-319-63245-2_17. Adv Exp Med Biol. 2017. PMID: 29047093 Review.
Cited by
-
Overview on Interactive Role of Inflammation, Reactive Oxygen Species, and Calcium Signaling in Asthma, COPD, and Pulmonary Hypertension.Adv Exp Med Biol. 2021;1304:147-164. doi: 10.1007/978-3-030-68748-9_9. Adv Exp Med Biol. 2021. PMID: 34019268
-
Functional Repercussions of Hypoxia-Inducible Factor-2α in Idiopathic Pulmonary Fibrosis.Cells. 2022 Sep 20;11(19):2938. doi: 10.3390/cells11192938. Cells. 2022. PMID: 36230900 Free PMC article. Review.
-
Rieske iron-sulfur protein induces FKBP12.6/RyR2 complex remodeling and subsequent pulmonary hypertension through NF-κB/cyclin D1 pathway.Nat Commun. 2020 Jul 15;11(1):3527. doi: 10.1038/s41467-020-17314-1. Nat Commun. 2020. PMID: 32669538 Free PMC article.
-
Hypoxia-Induced Mitochondrial ROS and Function in Pulmonary Arterial Endothelial Cells.Cells. 2024 Nov 1;13(21):1807. doi: 10.3390/cells13211807. Cells. 2024. PMID: 39513914 Free PMC article.
-
Isorhapontigenin ameliorates cerebral ischemia/reperfusion injury via modulating Kinase Cε/Nrf2/HO-1 signaling pathway.Brain Behav. 2021 Jul;11(7):e02143. doi: 10.1002/brb3.2143. Epub 2021 Jun 8. Brain Behav. 2021. PMID: 34102010 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

