Improving titer while maintaining quality of final formulated drug substance via optimization of CHO cell culture conditions in low-iron chemically defined media
- PMID: 29388872
- PMCID: PMC5916559
- DOI: 10.1080/19420862.2018.1433978
Improving titer while maintaining quality of final formulated drug substance via optimization of CHO cell culture conditions in low-iron chemically defined media
Abstract
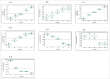
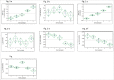
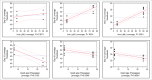
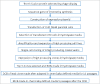
During biopharmaceutical process development, it is important to improve titer to reduce drug manufacturing costs and to deliver comparable quality attributes of therapeutic proteins, which helps to ensure patient safety and efficacy. We previously reported that relative high-iron concentrations in media increased titer, but caused unacceptable coloration of a fusion protein during early-phase process development. Ultimately, the fusion protein with acceptable color was manufactured using low-iron media, but the titer decreased significantly in the low-iron process. Here, long-term passaging in low-iron media is shown to significantly improve titer while maintaining acceptable coloration during late-phase process development. However, the long-term passaging also caused a change in the protein charge variant profile by significantly increasing basic variants. Thus, we systematically studied the effect of media components, seed culture conditions, and downstream processing on productivity and quality attributes. We found that removing β-glycerol phosphate (BGP) from basal media reduced basic variants without affecting titer. Our goals for late-phase process development, improving titer and matching quality attributes to the early-phase process, were thus achieved by prolonging seed culture age and removing BGP. This process was also successfully scaled up in 500-L bioreactors. In addition, we demonstrated that higher concentrations of reactive oxygen species were present in the high-iron Chinese hamster ovary cell cultures compared to that in the low-iron cultures, suggesting a possible mechanism for the drug substance coloration caused by high-iron media. Finally, hypotheses for the mechanisms of titer improvement by both high-iron and long-term culture are discussed.
Keywords: CHO long-term culture; basic variants; iron; manufacturing process development; protein drug substance color; titer, quality; β-glycerol phosphate.
Figures





Similar articles
-
Effect of cell culture medium components on color of formulated monoclonal antibody drug substance.Biotechnol Prog. 2013 Sep-Oct;29(5):1270-7. doi: 10.1002/btpr.1772. Epub 2013 Jun 27. Biotechnol Prog. 2013. PMID: 23804462
-
Effect of cell culture medium additives on color and acidic charge variants of a monoclonal antibody.Biotechnol Prog. 2018 Sep;34(5):1298-1307. doi: 10.1002/btpr.2668. Epub 2018 Aug 9. Biotechnol Prog. 2018. PMID: 29882320
-
Impact of hydrolysates on monoclonal antibody productivity, purification and quality in Chinese hamster ovary cells.J Biosci Bioeng. 2016 Oct;122(4):499-506. doi: 10.1016/j.jbiosc.2016.03.003. Epub 2016 Apr 7. J Biosci Bioeng. 2016. PMID: 27067279
-
Quantifying the impact of cell culture media on CHO cell growth and protein production.Biotechnol Adv. 2021 Sep-Oct;50:107761. doi: 10.1016/j.biotechadv.2021.107761. Epub 2021 May 1. Biotechnol Adv. 2021. PMID: 33945850 Review.
-
Consequences of trace metal variability and supplementation on Chinese hamster ovary (CHO) cell culture performance: A review of key mechanisms and considerations.Biotechnol Bioeng. 2019 Dec;116(12):3446-3456. doi: 10.1002/bit.27140. Epub 2019 Aug 30. Biotechnol Bioeng. 2019. PMID: 31403183 Review.
Cited by
-
Oxidative stress-alleviating strategies to improve recombinant protein production in CHO cells.Biotechnol Bioeng. 2020 Apr;117(4):1172-1186. doi: 10.1002/bit.27247. Epub 2019 Dec 20. Biotechnol Bioeng. 2020. PMID: 31814104 Free PMC article. Review.
-
Explainable AI for CHO cell culture media optimization and prediction of critical quality attribute.Appl Microbiol Biotechnol. 2024 Apr 24;108(1):308. doi: 10.1007/s00253-024-13147-w. Appl Microbiol Biotechnol. 2024. PMID: 38656382 Free PMC article.
-
Modulating cell culture oxidative stress reduces protein glycation and acidic charge variant formation.MAbs. 2019 Jan;11(1):205-216. doi: 10.1080/19420862.2018.1537533. Epub 2019 Jan 3. MAbs. 2019. PMID: 30602334 Free PMC article.
-
Exploring the limits of conventional small-scale CHO fed-batch for accelerated on demand monoclonal antibody production.Bioprocess Biosyst Eng. 2022 Feb;45(2):297-307. doi: 10.1007/s00449-021-02657-w. Epub 2021 Nov 9. Bioprocess Biosyst Eng. 2022. PMID: 34750672 Free PMC article.
-
Developments and opportunities in continuous biopharmaceutical manufacturing.MAbs. 2021 Jan-Dec;13(1):1903664. doi: 10.1080/19420862.2021.1903664. MAbs. 2021. PMID: 33843449 Free PMC article. Review.
References
-
- Watier H, Reichert JM. Evolution of antibody therapeutics. In: Vaughn T, Osbourn J, Jallal B, Protein Therapeutics. Weinheim, Germany: Wiley-VCH; 2017. p. 25–49.
-
- von Richter O, Skerjanec A, Afonso M, Sanguino Heinrich S, Poetzl J, Woehling H, Velinova M, Koch A, Kollins D, Macke L, et al. GP2015, a proposed etanercept biosimilar: Pharmacokinetic similarity to its reference product and comparison of its autoinjector device with prefilled syringes. Br J Clin Pharmacol. 2017;83(4):732–41. doi: 10.1111/bcp.13170. PMID:27790726. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
