In Vivo PET Assay of Tumor Glutamine Flux and Metabolism: In-Human Trial of 18F-(2S,4R)-4-Fluoroglutamine
- PMID: 29388903
- PMCID: PMC5929369
- DOI: 10.1148/radiol.2017162610
In Vivo PET Assay of Tumor Glutamine Flux and Metabolism: In-Human Trial of 18F-(2S,4R)-4-Fluoroglutamine
Abstract
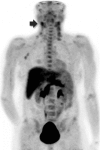
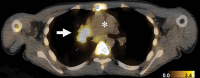
Purpose To assess the clinical safety, pharmacokinetics, and tumor imaging characteristics of fluorine 18-(2S,4R)-4-fluoroglutamine (FGln), a glutamine analog radiologic imaging agent. Materials and Methods This study was approved by the institutional review board and conducted under a U.S. Food and Drug Administration-approved Investigational New Drug application in accordance with the Helsinki Declaration and the Health Insurance Portability and Accountability Act. All patients provided written informed consent. Between January 2013 and October 2016, 25 adult patients with cancer received an intravenous bolus of FGln tracer (mean, 244 MBq ± 118, <100 μg) followed by positron emission tomography (PET) and blood radioassays. Patient data were summarized with descriptive statistics. FGln biodistribution and plasma amino acid levels in nonfasting patients (n = 13) were compared with those from patients who fasted at least 8 hours before injection (n = 12) by using nonparametric one-way analysis of variance with Bonferroni correction. Tumor FGln avidity versus fluorodeoxyglucose (FDG) avidity in patients with paired PET scans (n = 15) was evaluated with the Fisher exact test. P < .05 was considered indicative of a statistically significant difference. Results FGln PET depicted tumors of different cancer types (breast, pancreas, renal, neuroendocrine, lung, colon, lymphoma, bile duct, or glioma) in 17 of the 25 patients, predominantly clinically aggressive tumors with genetic mutations implicated in abnormal glutamine metabolism. Acute fasting had no significant effect on FGln biodistribution and plasma amino acid levels. FGln-avid tumors were uniformly FDG-avid but not vice versa (P = .07). Patients experienced no adverse effects. Conclusion Preliminary human FGln PET trial results provide clinical validation of abnormal glutamine metabolism as a potential tumor biomarker for targeted radiotracer imaging in several different cancer types. © RSNA, 2018 Online supplemental material is available for this article. Clinical trial registration no. NCT01697930.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

