Methods for efficient analysis of tocopherols, tocotrienols and their metabolites in animal samples with HPLC-EC
- PMID: 29389570
- PMCID: PMC9332665
- DOI: 10.1016/j.jfda.2017.07.012
Methods for efficient analysis of tocopherols, tocotrienols and their metabolites in animal samples with HPLC-EC
Abstract
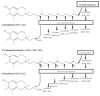
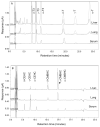
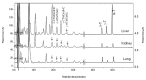
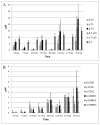
Tocopherols and tocotrienols, collectively known as vitamin E, have received a great deal of attention because of their interesting biological activities. In the present study, we reexamined and improved previous methods of sample preparation and the conditions of high-performance liquid chromatography for more accurate quantification of tocopherols, tocotrienols and their major chain-degradation metabolites. For the analysis of serum tocopherols/tocotrienols, we reconfirmed our method of mixing serum with ethanol followed by hexane extraction. For the analysis of tissue samples, we improved our methods by extracting tocopherols/tocotrienols directly from tissue homogenate with hexane. For the analysis of total amounts (conjugated and unconjugated forms) of side-chain degradation metabolites, the samples need to be deconjugated by incubating with β-glucuronidase and sulfatase; serum samples can be directly used for the incubation, whereas for tissue homogenates a pre-deproteination step is needed. The present methods are sensitive, convenient and are suitable for the determination of different forms of vitamin E and their metabolites in animal and human studies. Results from the analysis of serum, liver, kidney, lung and urine samples from mice that had been treated with mixtures of tocotrienols and tocopherols are presented as examples.
Keywords: Metabolites; Mice; Tissue levels; Tocopherols; Tocotrienols.
Copyright © 2017. Published by Elsevier B.V.
Figures

Similar articles
-
Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans.J Agric Food Chem. 2010 Apr 28;58(8):4844-52. doi: 10.1021/jf904464u. J Agric Food Chem. 2010. PMID: 20222730 Free PMC article.
-
Development and optimization of ultra-high performance supercritical fluid chromatography mass spectrometry method for high-throughput determination of tocopherols and tocotrienols in human serum.Anal Chim Acta. 2016 Aug 31;934:252-65. doi: 10.1016/j.aca.2016.06.008. Epub 2016 Jun 9. Anal Chim Acta. 2016. PMID: 27506367
-
GC-MS and LC-MS approaches for determination of tocopherols and tocotrienols in biological and food matrices.J Pharm Biomed Anal. 2016 Aug 5;127:156-69. doi: 10.1016/j.jpba.2016.02.051. Epub 2016 Mar 2. J Pharm Biomed Anal. 2016. PMID: 26964480 Review.
-
Combination of saponification and dispersive liquid-liquid microextraction for the determination of tocopherols and tocotrienols in cereals by reversed-phase high-performance liquid chromatography.J Chromatogr A. 2013 Jul 26;1300:31-7. doi: 10.1016/j.chroma.2013.03.036. Epub 2013 Mar 25. J Chromatogr A. 2013. PMID: 23587317
-
Chromatographic Separation of Vitamin E Enantiomers.Molecules. 2017 Feb 4;22(2):233. doi: 10.3390/molecules22020233. Molecules. 2017. PMID: 28165404 Free PMC article. Review.
Cited by
-
Oxidative status of a yogurt-like fermented maize product containing phytosterols.J Food Sci Technol. 2018 May;55(5):1859-1869. doi: 10.1007/s13197-018-3102-5. Epub 2018 Mar 19. J Food Sci Technol. 2018. PMID: 29666539 Free PMC article.
-
Plasma, Prostate and Urine Levels of Tocopherols and Metabolites in Men after Supplementation with a γ-Tocopherol-Rich Vitamin E Mixture.Nutr Cancer. 2021;73(11-12):2740-2750. doi: 10.1080/01635581.2020.1857412. Epub 2020 Dec 15. Nutr Cancer. 2021. PMID: 33319628 Free PMC article. Clinical Trial.
-
α-Tocomonoenol Is Bioavailable in Mice and May Partly Be Regulated by the Function of the Hepatic α‑Tocopherol Transfer Protein.Molecules. 2020 Oct 19;25(20):4803. doi: 10.3390/molecules25204803. Molecules. 2020. PMID: 33086686 Free PMC article.
References
-
- Traber MG, Vitamin E. In: Modern nutrition in health and disease. 10th ed. Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 396–411.
-
- Birringer M, Drogan D, Brigelius-Flohe R. Tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic Biol Med. 2001;31:226–32. [in Eng] - PubMed
-
- Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. Biol Chem. 2002;277:25290–6. [in Eng] - PubMed
-
- Brigelius-Flohe R. Vitamin E and drug metabolism. Biochem Biophys Res Comm. 2003;305:737–40. [in Eng] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
