Development and validation of an LC-MS/MS method for quantification of NC-8 in rat plasma and its application to pharmacokinetic studies
- PMID: 29389580
- PMCID: PMC9332635
- DOI: 10.1016/j.jfda.2017.09.003
Development and validation of an LC-MS/MS method for quantification of NC-8 in rat plasma and its application to pharmacokinetic studies
Abstract
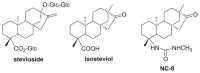
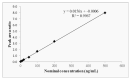
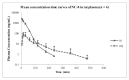
ent-16-Oxobeyeran-19-N-methylureido (NC-8) is a recently synthesized derivative of isosteviol that showed anti-hepatitis B virus (HBV) activity by disturbing replication and gene expression of the HBV and by inhibiting the host toll-like receptor 2/nuclear factor-κB signaling pathway. To study its pharmacokinetics as a part of the drug development process, a highly sensitive, rapid, and reliable liquid chromatography tandem mass spectrometry (LC-MS/MS) method was developed and validated for determining NC-8 in rat plasma. After protein precipitation extraction, the chromatographic separation of the analyte and internal standard (IS; diclofenac sodium) was performed on a reverse-phase Luna C18 column coupled with a Quattro Ultima triple quadruple mass spectrometer in the multiple-reaction monitoring mode using the transitions, m/z 347.31 → 75.09 for NC-8 and m/z 295.89 → 214.06 for the IS. The lower limit of quantitation was 0.5 ng/mL. The linear scope of the standard curve was between 0.5 and 500 ng/mL. Both the precision (coefficient of variation; %) and accuracy (relative error; %) were within acceptable criteria of <15%. Recoveries ranged from 104% to 113.4%, and the matrix effects (absolute) were non-significant (CV ≤ 6%). The validated method was successfully applied to investigate the pharmacokinetics of NC-8 in male Sprague-Dawley rats. The present methodology provides an analytical means to better understand the preliminary pharmacokinetics of NC-8 for investigations on further drug development.
Keywords: Isosteviol derivative; LC–MS/MS; NC-8; Pharmacokinetics.
Copyright © 2017. Published by Elsevier B.V.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures


Similar articles
-
Simultaneous quantification of Kirenol and ent-16β,17-dihydroxy-kauran-19-oic acid from Herba Siegesbeckiae in rat plasma by liquid chromatography-tandem mass spectrometry and its application to pharmacokinetic studies.J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Oct 15;937:18-24. doi: 10.1016/j.jchromb.2013.08.019. Epub 2013 Aug 19. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 24008120
-
Oral and i.v. pharmacokinetics of isosteviol in rats as assessed by a new sensitive LC-MS/MS method.J Pharm Biomed Anal. 2008 Nov 4;48(3):986-90. doi: 10.1016/j.jpba.2008.06.015. Epub 2008 Jul 3. J Pharm Biomed Anal. 2008. PMID: 18701231
-
Synthesis and antiviral effects of isosteviol-derived analogues against the hepatitis B virus.Phytochemistry. 2014 Mar;99:107-14. doi: 10.1016/j.phytochem.2013.12.014. Epub 2014 Jan 23. Phytochemistry. 2014. PMID: 24461778
-
Validation of an UHPLC-MS/MS Method for the Determination of Glaucocalyxin A, a Novel Potent Negative Akt Regulator in Rat Plasma, Lung and Brain Tissues: Application to a Pharmacokinetic Study.J Chromatogr Sci. 2020 Apr 22;58(3):234-240. doi: 10.1093/chromsci/bmz095. J Chromatogr Sci. 2020. PMID: 31711232
-
Development and validation of an LC-MS/MS method for the determination of a novel thienoquinolin urea transporter inhibitor PU-48 in rat plasma and its application to a pharmacokinetic study.Biomed Chromatogr. 2018 Apr;32(4). doi: 10.1002/bmc.4157. Epub 2017 Dec 22. Biomed Chromatogr. 2018. PMID: 29193233
Cited by
-
Zinc-Boron Complex-Based Dietary Supplements for Longevity and Healthy Life.Curr Health Sci J. 2023 Jul-Sep;49(3):381-387. doi: 10.12865/CHSJ.49.03.10. Epub 2023 Sep 30. Curr Health Sci J. 2023. PMID: 38314211 Free PMC article.
-
Chinese medicine in the treatment of chronic hepatitis B: The mechanisms of signal pathway regulation.Heliyon. 2024 Oct 12;10(20):e39176. doi: 10.1016/j.heliyon.2024.e39176. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640799 Free PMC article. Review.
-
Pharmacokinetic Properties of Acetyl Tributyl Citrate, a Pharmaceutical Excipient.Pharmaceutics. 2018 Oct 8;10(4):177. doi: 10.3390/pharmaceutics10040177. Pharmaceutics. 2018. PMID: 30297635 Free PMC article.
-
A Study of Opiate, Opiate Metabolites and Antihistamines in Urine after Consumption of Cold Syrups by LC-MS/MS.Molecules. 2020 Feb 21;25(4):972. doi: 10.3390/molecules25040972. Molecules. 2020. PMID: 32098143 Free PMC article.
References
-
- Word Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015 March; - PubMed
-
- Lahlou M. The success of natural products in drug discovery. Pharmacol Pharm. 2013;4:17–31.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
