A controlled human malaria infection model enabling evaluation of transmission-blocking interventions
- PMID: 29389671
- PMCID: PMC5873858
- DOI: 10.1172/JCI98012
A controlled human malaria infection model enabling evaluation of transmission-blocking interventions
Abstract
Background: Drugs and vaccines that can interrupt the transmission of Plasmodium falciparum will be important for malaria control and elimination. However, models for early clinical evaluation of candidate transmission-blocking interventions are currently unavailable. Here, we describe a new model for evaluating malaria transmission from humans to Anopheles mosquitoes using controlled human malaria infection (CHMI).
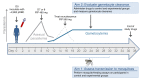
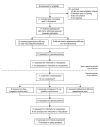
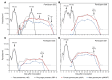
Methods: Seventeen healthy malaria-naive volunteers underwent CHMI by intravenous inoculation of P. falciparum-infected erythrocytes to initiate blood-stage infection. Seven to eight days after inoculation, participants received piperaquine (480 mg) to attenuate asexual parasite replication while allowing gametocytes to develop and mature. Primary end points were development of gametocytemia, the transmissibility of gametocytes from humans to mosquitoes, and the safety and tolerability of the CHMI transmission model. To investigate in vivo gametocytocidal drug activity in this model, participants were either given an experimental antimalarial, artefenomel (500 mg), or a known gametocytocidal drug, primaquine (15 mg), or remained untreated during the period of gametocyte carriage.
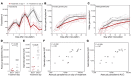
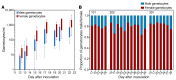
Results: Male and female gametocytes were detected in all participants, and transmission to mosquitoes was achieved from 8 of 11 (73%) participants evaluated. Compared with results in untreated controls (n = 7), primaquine (15 mg, n = 5) significantly reduced gametocyte burden (P = 0.01), while artefenomel (500 mg, n = 4) had no effect. Adverse events (AEs) were mostly mild or moderate. Three AEs were assessed as severe - fatigue, elevated alanine aminotransferase, and elevated aspartate aminotransferase - and were attributed to malaria infection. Transaminase elevations were transient, asymptomatic, and resolved without intervention.
Conclusion: We report the safe and reproducible induction of P. falciparum gametocytes in healthy malaria-naive volunteers at densities infectious to mosquitoes, thereby demonstrating the potential for evaluating transmission-blocking interventions in this model.
Trial registration: ClinicalTrials.gov NCT02431637 and NCT02431650.
Funding: Bill & Melinda Gates Foundation.
Keywords: Clinical Trials; Infectious disease; Malaria.
Conflict of interest statement
Figures



Comment in
-
What goes around comes around: modeling malaria transmission from humans back to mosquitos.J Clin Invest. 2018 Apr 2;128(4):1264-1266. doi: 10.1172/JCI120260. Epub 2018 Mar 12. J Clin Invest. 2018. PMID: 29528336 Free PMC article.
References
-
- World Health Organization. World Malaria Report 2016. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report... Accessed February 12, 2018.
-
- Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malariatherapy data. Trans R Soc Trop Med Hyg. 2001;95(5):497–501. doi: 10.1016/S0035-9203(01)90016-1. - DOI - PubMed
-
- Sinden RE. Sexual development of malarial parasites. Adv Parasitol. 1983;22:153–216. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

