Fermentability of Novel Type-4 Resistant Starches in In Vitro System
- PMID: 29389870
- PMCID: PMC5848122
- DOI: 10.3390/foods7020018
Fermentability of Novel Type-4 Resistant Starches in In Vitro System
Abstract
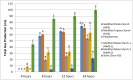
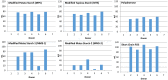
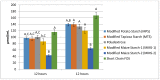
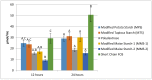
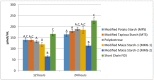
Resistant starches are non-digestible starches that are fermented in the colon by microbiota. These carbohydrates are prebiotic and can be beneficial to consumer health. Many types of resistant starch exist with varying physical properties that may result in differences in fermentability. The objective of this research project was to compare potential prebiotic effects and fermentability of four novel resistant starches using an in vitro fermentation system and measuring changes in total gas production, pH, and formation of SCFAs (short chain fatty acids). Fecal donations were collected from seven healthy volunteers. Four novel resistant starches, modified potato starch (MPS), modified tapioca starch (MTS), and modified maize starches (MMS-1 and MMS-2), were analyzed and compared to polydextrose and short chain fructooligosaccharides (FOS) as controls. After twenty-four hours of fermentation, MPS and MTS responded similarly in gas production (74 mL; 70.6 mL respectively), pH (5.93; 5.93 respectively), and SCFA production (Acetate: 115; 124, Propionate: 21; 26, Butyrate: 29; 31 μmol/mL respectively). While MMS-1 had similar gas production and individual SCFA production, the pH was significantly higher (6.06). The fermentation of MMS-2 produced the least amount of gas (22 mL), with a higher pH (6.34), and lower acetate production (78.4 μmol/mL). All analyzed compounds were fermentable and promoted the formation of beneficial SCFAs.
Keywords: dietary fiber; prebiotics; resistant starch.
Conflict of interest statement
M.L.S. is an employee of Ingredion Incorporated. J.L.S., J.M.E. and J.L.C. declare no conflict of interest.
Figures



References
-
- United States Food and Drug Administration . Scientific Evaluation of the Evidence on the Beneficial Physiological Effects of Isolated or Synthetic Non-DiGestible Carbohydrates Submitted as a Citizen Petition (21 CFR 10.30): Guidance for Industry. United States Food and Drug Administration; Silver Spring, MD, USA: 2016.
-
- Office of Nutrition and Food Labeling. Center for Food Safety and Applied Nutrition. United States Food and Drug Administration. US Department of Health and Human Services . Science Review of Isolated and Synthetic Non-Digestible Carbohydrates. United States Food and Drug Administration; Silver Spring, MD, USA: 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources

