When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma
- PMID: 29389898
- PMCID: PMC5855658
- DOI: 10.3390/ijms19020436
When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma
Abstract
As a substantial part of the brain tumor microenvironment (TME), glioma-associated microglia/macrophages (GAMs) have an emerging role in tumor progression and in controlling anti-tumor immune responses. We review challenges and improvements of cell models and highlight the contribution of this highly plastic cell population to an immunosuppressive TME, besides their well-known functional role regarding glioma cell invasion and angiogenesis. Finally, we summarize first therapeutic interventions to target GAMs and their effect on the immunobiology of gliomas, focusing on their interaction with T cells.
Keywords: GAMs; GBM; TME; glioblastoma; glioma; glioma-associated microglia/macrophages; microglia; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
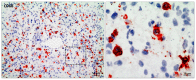

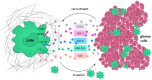
Similar articles
-
Role of myeloid cells in the immunosuppressive microenvironment in gliomas.Immunobiology. 2020 Jan;225(1):151853. doi: 10.1016/j.imbio.2019.10.002. Epub 2019 Oct 19. Immunobiology. 2020. PMID: 31703822 Review.
-
Molecular interactions between tumor and its microenvironment in malignant gliomas.Postepy Biochem. 2018 Oct 15;64(2):129-140. doi: 10.18388/pb.2018_123. Postepy Biochem. 2018. PMID: 30656895 Review. English.
-
The roles of glioma-associated macrophages/microglia and potential targets for anti-glioma therapy.Immunol Med. 2025 Mar;48(1):24-32. doi: 10.1080/25785826.2024.2411035. Epub 2024 Oct 11. Immunol Med. 2025. PMID: 39391957 Review.
-
[The immunosuppressive microenvironment of malignant gliomas].Arkh Patol. 2015 Nov-Dec;77(6):54-63. doi: 10.17116/patol201577654-63. Arkh Patol. 2015. PMID: 26841651 Review. Russian.
-
Distribution and prognostic impact of microglia/macrophage subpopulations in gliomas.Brain Pathol. 2019 Jul;29(4):513-529. doi: 10.1111/bpa.12690. Epub 2019 Jan 15. Brain Pathol. 2019. PMID: 30506802 Free PMC article.
Cited by
-
Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology.Neuron. 2019 Nov 6;104(3):442-449. doi: 10.1016/j.neuron.2019.08.028. Neuron. 2019. PMID: 31697921 Free PMC article. Review.
-
Interaction of glioma-associated microglia/macrophages and anti-PD1 immunotherapy.Cancer Immunol Immunother. 2023 Jun;72(6):1685-1698. doi: 10.1007/s00262-022-03358-3. Epub 2023 Jan 9. Cancer Immunol Immunother. 2023. PMID: 36624155 Free PMC article.
-
Advanced Biomaterials for Cell-Specific Modulation and Restore of Cancer Immunotherapy.Adv Sci (Weinh). 2022 May;9(16):e2200027. doi: 10.1002/advs.202200027. Epub 2022 Mar 27. Adv Sci (Weinh). 2022. PMID: 35343112 Free PMC article. Review.
-
Friends with Benefits: Chemokines, Glioblastoma-Associated Microglia/Macrophages, and Tumor Microenvironment.Int J Mol Sci. 2022 Feb 24;23(5):2509. doi: 10.3390/ijms23052509. Int J Mol Sci. 2022. PMID: 35269652 Free PMC article. Review.
-
Is There Any Evidence of Monocytes Involvement in Alzheimer's Disease? A Pilot Study on Human Postmortem Brain.J Alzheimers Dis Rep. 2021 Dec 23;5(1):887-897. doi: 10.3233/ADR-210052. eCollection 2021. J Alzheimers Dis Rep. 2021. PMID: 35088038 Free PMC article.
References
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

