High Levels of Dual-Class Drug Resistance in HIV-Infected Children Failing First-Line Antiretroviral Therapy in Southern Ethiopia
- PMID: 29389912
- PMCID: PMC5850367
- DOI: 10.3390/v10020060
High Levels of Dual-Class Drug Resistance in HIV-Infected Children Failing First-Line Antiretroviral Therapy in Southern Ethiopia
Abstract
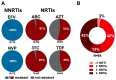
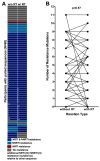
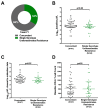
Clinical monitoring of pediatric HIV treatment remains a major challenge in settings where drug resistance genotyping is not routinely available. As a result, our understanding of drug resistance, and its impact on subsequent therapeutic regimens available in these settings, remains limited. We investigate the prevalence and correlates of HIV-1 drug resistance among 94 participants of the Ethiopia Pediatric HIV Cohort failing first-line combination antiretroviral therapy (cART) using dried blood spot-based genotyping. Overall, 81% (73/90) of successfully genotyped participants harbored resistance mutations, including 69% (62/90) who harbored resistance to both Nucleoside Reverse Transcriptase Inhibitors (NRTIs) and Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs). Strikingly, 42% of resistant participants harbored resistance to all four NRTIs recommended for second-line use in this setting, meaning that there are effectively no remaining cART options for these children. Longer cART duration and prior regimen changes were significantly associated with detection of drug resistance mutations. Replicate genotyping increased the breadth of drug resistance detected in 34% of cases, and thus is recommended for consideration when typing from blood spots. Implementation of timely drug resistance testing and access to newer antiretrovirals and drug classes are urgently needed to guide clinical decision-making and improve outcomes for HIV-infected children on first-line cART in Ethiopia.
Keywords: Ethiopia; HIV; children; dried blood spots; drug resistance; first-line combination antiretroviral therapy (cART); genotyping; pediatrics; treatment failure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Prevalence and Correlates of Pre-Treatment HIV Drug Resistance among HIV-Infected Children in Ethiopia.Viruses. 2019 Sep 19;11(9):877. doi: 10.3390/v11090877. Viruses. 2019. PMID: 31546824 Free PMC article.
-
Low Incidence of HIV-1C Acquired Drug Resistance 10 Years after Roll-Out of Antiretroviral Therapy in Ethiopia: A Prospective Cohort Study.PLoS One. 2015 Oct 29;10(10):e0141318. doi: 10.1371/journal.pone.0141318. eCollection 2015. PLoS One. 2015. PMID: 26512902 Free PMC article.
-
Viral Suppression and HIV Drug Resistance Among Patients on Second-Line Antiretroviral Therapy in Selected Health Facility in Ethiopia.Viruses. 2025 Jan 31;17(2):206. doi: 10.3390/v17020206. Viruses. 2025. PMID: 40006960 Free PMC article.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Management of paediatric HIV-1 resistance.Curr Opin Infect Dis. 2009 Jun;22(3):256-63. doi: 10.1097/qco.0b013e3283298f1f. Curr Opin Infect Dis. 2009. PMID: 19405216 Free PMC article. Review.
Cited by
-
Global, regional, and national prevalence of HIV-1 drug resistance in treatment-naive and treatment-experienced children and adolescents: a systematic review and meta-analysis.EClinicalMedicine. 2024 Oct 4;77:102859. doi: 10.1016/j.eclinm.2024.102859. eCollection 2024 Nov. EClinicalMedicine. 2024. PMID: 39430612 Free PMC article.
-
HIV-1 resistance mutations and genetic diversity among children failing antiretroviral treatment in five healthcare facilities in Benin, West Africa.PLoS One. 2025 Jan 29;20(1):e0317882. doi: 10.1371/journal.pone.0317882. eCollection 2025. PLoS One. 2025. PMID: 39879199 Free PMC article.
-
HIV-1 prevalence, drug resistance, and associated factors in the urban Ethiopian population.Sci Rep. 2025 May 17;15(1):17216. doi: 10.1038/s41598-025-02122-8. Sci Rep. 2025. PMID: 40382412 Free PMC article.
-
Rates and Correlates of Short Term Virologic Response among Treatment-Naïve HIV-Infected Children Initiating Antiretroviral Therapy in Ethiopia: A Multi-Center Prospective Cohort Study.Pathogens. 2019 Sep 24;8(4):161. doi: 10.3390/pathogens8040161. Pathogens. 2019. PMID: 31554200 Free PMC article.
-
Full-spectrum HIV drug resistance mutation detection by high-resolution complete pol gene sequencing.J Clin Virol. 2023 Jul;164:105491. doi: 10.1016/j.jcv.2023.105491. Epub 2023 May 6. J Clin Virol. 2023. PMID: 37182384 Free PMC article.
References
-
- The Joint United Nations Programme on HIV/AIDS (UNAIDS) Fact Sheet—Global HIV Statistics. UNAIDS Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2017.
-
- The Joint United Nations Programme on HIV/AIDS (UNAIDS) Global AIDS Update. UNAIDS Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2016. - PubMed
-
- Federal Ministry of Health Ethiopian Public Health Institute . HIV Related Estimates and Projections for Ethiopia-2017. Public Health Institute, Ethiopian Federal Ministry of Health; Addis Ababa, Ethiopia: 2017.
-
- Resino S., Bellon J.M., Resino R., Navarro M.L., Tomas Ramos J., de Jose M.I., Mellado M.J., Munoz-Fernandez M.A. Extensive implementation of highly active antiretroviral therapy shows great effect on survival and surrogate markers in vertically HIV-infected children. Clin. Infect. Dis. 2004;38:1605–1612. doi: 10.1086/420738. - DOI - PubMed
-
- Berk D.R., Falkovitz-Halpern M.S., Hill D.W., Albin C., Arrieta A., Bork J.M., Cohan D., Nilson B., Petru A., Ruiz J., et al. Temporal trends in early clinical manifestations of perinatal HIV infection in a population-based cohort. JAMA. 2005;293:2221–2231. doi: 10.1001/jama.293.18.2221. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

