Inferring pregnancy episodes and outcomes within a network of observational databases
- PMID: 29389968
- PMCID: PMC5794136
- DOI: 10.1371/journal.pone.0192033
Inferring pregnancy episodes and outcomes within a network of observational databases
Abstract
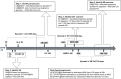
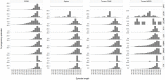
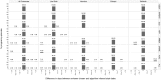
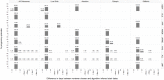
Administrative claims and electronic health records are valuable resources for evaluating pharmaceutical effects during pregnancy. However, direct measures of gestational age are generally not available. Establishing a reliable approach to infer the duration and outcome of a pregnancy could improve pharmacovigilance activities. We developed and applied an algorithm to define pregnancy episodes in four observational databases: three US-based claims databases: Truven MarketScan® Commercial Claims and Encounters (CCAE), Truven MarketScan® Multi-state Medicaid (MDCD), and the Optum ClinFormatics® (Optum) database and one non-US database, the United Kingdom (UK) based Clinical Practice Research Datalink (CPRD). Pregnancy outcomes were classified as live births, stillbirths, abortions and ectopic pregnancies. Start dates were estimated using a derived hierarchy of available pregnancy markers, including records such as last menstrual period and nuchal ultrasound dates. Validation included clinical adjudication of 700 electronic Optum and CPRD pregnancy episode profiles to assess the operating characteristics of the algorithm, and a comparison of the algorithm's Optum pregnancy start estimates to starts based on dates of assisted conception procedures. Distributions of pregnancy outcome types were similar across all four data sources and pregnancy episode lengths found were as expected for all outcomes, excepting term lengths in episodes that used amenorrhea and urine pregnancy tests for start estimation. Validation survey results found highest agreement between reviewer chosen and algorithm operating characteristics for questions assessing pregnancy status and accuracy of outcome category with 99-100% agreement for Optum and CPRD. Outcome date agreement within seven days in either direction ranged from 95-100%, while start date agreement within seven days in either direction ranged from 90-97%. In Optum validation sensitivity analysis, a total of 73% of algorithm estimated starts for live births were in agreement with fertility procedure estimated starts within two weeks in either direction; ectopic pregnancy 77%, stillbirth 47%, and abortion 36%. An algorithm to infer live birth and ectopic pregnancy episodes and outcomes can be applied to multiple observational databases with acceptable accuracy for further epidemiologic research. Less accuracy was found for start date estimations in stillbirth and abortion outcomes in our sensitivity analysis, which may be expected given the nature of the outcomes.
Conflict of interest statement
Figures


Similar articles
-
Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink.Vaccine. 2013 Jun 12;31(27):2898-903. doi: 10.1016/j.vaccine.2013.03.069. Epub 2013 Apr 30. Vaccine. 2013. PMID: 23639917
-
Development of an algorithm to identify pregnancy episodes and related outcomes in health care claims databases: An application to antiepileptic drug use in 4.9 million pregnant women in France.Pharmacoepidemiol Drug Saf. 2018 Jul;27(7):763-770. doi: 10.1002/pds.4556. Epub 2018 May 15. Pharmacoepidemiol Drug Saf. 2018. PMID: 29763992 Free PMC article.
-
Optimizing an algorithm for the identification and classification of pregnancy outcomes in German claims data.Pharmacoepidemiol Drug Saf. 2018 Sep;27(9):1005-1010. doi: 10.1002/pds.4588. Epub 2018 Jul 18. Pharmacoepidemiol Drug Saf. 2018. PMID: 30022557
-
Beginning and duration of pregnancy in automated health care databases: review of estimation methods and validation results.Pharmacoepidemiol Drug Saf. 2015 Apr;24(4):335-42. doi: 10.1002/pds.3743. Epub 2015 Jan 28. Pharmacoepidemiol Drug Saf. 2015. PMID: 25627986 Review.
-
Administrative Claims Data Versus Augmented Pregnancy Data for the Study of Pharmaceutical Treatments in Pregnancy.Curr Epidemiol Rep. 2017;4(2):106-116. doi: 10.1007/s40471-017-0104-1. Epub 2017 Apr 18. Curr Epidemiol Rep. 2017. PMID: 29399433 Free PMC article. Review.
Cited by
-
Data resource profile: Scottish Linked Pregnancy and Baby Dataset (SLiPBD).Int J Popul Data Sci. 2024 Jul 17;9(2):2390. doi: 10.23889/ijpds.v9i2.2390. eCollection 2024. Int J Popul Data Sci. 2024. PMID: 40151429 Free PMC article.
-
Association of platinum-based chemotherapy with live birth and infertility in female survivors of adolescent and young adult cancer.Fertil Steril. 2024 Jun;121(6):1020-1030. doi: 10.1016/j.fertnstert.2024.01.039. Epub 2024 Feb 3. Fertil Steril. 2024. PMID: 38316209 Free PMC article.
-
Early Pregnancy Loss Management in the Emergency Department vs Outpatient Setting.JAMA Netw Open. 2023 Mar 1;6(3):e232639. doi: 10.1001/jamanetworkopen.2023.2639. JAMA Netw Open. 2023. PMID: 36920395 Free PMC article.
-
Expanding the OMOP Common Data Model to Support Perinatal Research in Network Studies.Pharmacoepidemiol Drug Saf. 2025 Feb;34(2):e70106. doi: 10.1002/pds.70106. Pharmacoepidemiol Drug Saf. 2025. PMID: 39950235 Free PMC article.
-
Using national in vitro fertilization registries to validate clinical outcomes after in vitro fertilization covered by health insurance.Fertil Steril. 2025 Jun;123(6):1029-1038. doi: 10.1016/j.fertnstert.2024.12.015. Epub 2024 Dec 12. Fertil Steril. 2025. PMID: 39674336
References
-
- Hornbrook MC, Whitlock EP, Berg CJ, Callaghan WM, Bachman DJ, Gold R et al. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health services research. 2007;42(2):908–27. doi: 10.1111/j.1475-6773.2006.00635.x - DOI - PMC - PubMed
-
- Andrade SE, Raebel MA, Morse AN, Davis RL, Chan KA, Finkelstein JA et al. Use of prescription medications with a potential for fetal harm among pregnant women. Pharmacoepidemiology and drug safety. 2006;15(8):546–54. doi: 10.1002/pds.1235 - DOI - PubMed
-
- Hardy JR, Holford TR, Hall GC, Bracken MB. Strategies for identifying pregnancies in the automated medical records of the General Practice Research Database. Pharmacoepidemiology and drug safety. 2004;13(11):749–59. doi: 10.1002/pds.935 - DOI - PubMed
-
- Devine S, West S, Andrews E, Tennis P, Hammad TA, Eaton S et al. The identification of pregnancies within the general practice research database. Pharmacoepidemiology and drug safety. 2010;19(1):45–50. doi: 10.1002/pds.1862 - DOI - PubMed
-
- Cea-Soriano L, Garcia Rodriguez LA, Fernandez Cantero O, Hernandez-Diaz S. Challenges of using primary care electronic medical records in the UK to study medications in pregnancy. Pharmacoepidemiology and drug safety. 2013;22(9):977–85. doi: 10.1002/pds.3472 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

