Rhodopsin optogenetic toolbox v2.0 for light-sensitive excitation and inhibition in Caenorhabditis elegans
- PMID: 29389997
- PMCID: PMC5794093
- DOI: 10.1371/journal.pone.0191802
Rhodopsin optogenetic toolbox v2.0 for light-sensitive excitation and inhibition in Caenorhabditis elegans
Abstract
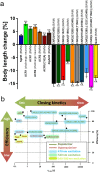
In optogenetics, rhodopsins were established as light-driven tools to manipulate neuronal activity. However, during long-term photostimulation using channelrhodopsin (ChR), desensitization can reduce effects. Furthermore, requirement for continuous presence of the chromophore all-trans retinal (ATR) in model systems lacking sufficient endogenous concentrations limits its applicability. We tested known, and engineered and characterized new variants of de- and hyperpolarizing rhodopsins in Caenorhabditis elegans. ChR2 variants combined previously described point mutations that may synergize to enable prolonged stimulation. Following brief light pulses ChR2(C128S;H134R) induced muscle activation for minutes or even for hours ('Quint': ChR2(C128S;L132C;H134R;D156A;T159C)), thus featuring longer open state lifetime than previously described variants. Furthermore, stability after ATR removal was increased compared to the step-function opsin ChR2(C128S). The double mutants C128S;H134R and H134R;D156C enabled increased effects during repetitive stimulation. We also tested new hyperpolarizers (ACR1, ACR2, ACR1(C102A), ZipACR). Particularly ACR1 and ACR2 showed strong effects in behavioral assays and very large currents with fast kinetics. In sum, we introduce highly light-sensitive optogenetic tools, bypassing previous shortcomings, and thus constituting new tools that feature high effectiveness and fast kinetics, allowing better repetitive stimulation or investigating prolonged neuronal activity states in C. elegans and, possibly, other systems.
Conflict of interest statement
Figures



References
-
- Knöpfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30(45):14998–5004. doi: 10.1523/JNEUROSCI.4190-10.2010 - DOI - PMC - PubMed
-
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004 - DOI - PubMed
-
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18(9):1213–25. doi: 10.1038/nn.4091 - DOI - PMC - PubMed
-
- Kocabas A, Shen CH, Guo ZV, Ramanathan S. Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature. 2012;490(7419):273–7. doi: 10.1038/nature11431 - DOI - PMC - PubMed
-
- Erbguth K, Prigge M, Schneider F, Hegemann P, Gottschalk A. Bimodal activation of different neuron classes with the spectrally red-shifted channelrhodopsin chimera C1V1 in Caenorhabditis elegans. PLoS ONE. 2012;7(10):e46827 doi: 10.1371/journal.pone.0046827 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

