Serum amyloid A3 is required for normal weight and immunometabolic function in mice
- PMID: 29390039
- PMCID: PMC5794179
- DOI: 10.1371/journal.pone.0192352
Serum amyloid A3 is required for normal weight and immunometabolic function in mice
Abstract
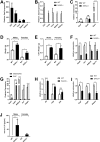
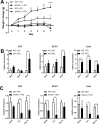
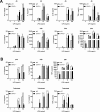
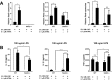
Serum amyloid A (SAA) is an apolipoprotein that is robustly upregulated in numerous inflammatory diseases and has been implicated as a candidate pro-inflammatory mediator. However, studies comparing endogenous SAAs and recombinant forms of the acute phase protein have generated conflicting data on the function of SAA in immunity. We generated SAA3 knockout mice to evaluate the contribution of SAA3 to immune-mediated disease, and found that mice lacking SAA3 develop adult-onset obesity and metabolic dysfunction along with defects in innate immune development. Mice that lack SAA3 gain more weight, exhibit increased visceral adipose deposition, and develop hepatic steatosis compared to wild-type littermates. Leukocytes from the adipose tissue of SAA3-/- mice express a pro-inflammatory phenotype, and bone marrow derived dendritic cells from mice lacking SAA3 secrete increased levels of IL-1β, IL-6, IL-23, and TNFα in response to LPS compared to cells from wild-type mice. Finally, BMDC lacking SAA3 demonstrate an impaired endotoxin tolerance response and inhibited responses to retinoic acid. Our findings indicate that endogenous SAA3 modulates metabolic and immune homeostasis.
Conflict of interest statement
Figures

References
-
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265(2):501–23. . - PubMed
-
- Steel DM, Sellar GC, Uhlar CM, Simon S, DeBeer FC, Whitehead AS. A constitutively expressed serum amyloid A protein gene (SAA4) is closely linked to, and shares structural similarities with, an acute-phase serum amyloid A protein gene (SAA2). Genomics. 1993;16(2):447–54. doi: 10.1006/geno.1993.1209 . - DOI - PubMed
-
- Ramadori G, Sipe JD, Colten HR. Expression and regulation of the murine serum amyloid A (SAA) gene in extrahepatic sites. J Immunol. 1985;135(6):3645–7. . - PubMed
-
- Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, et al. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187(1):64–73. doi: 10.4049/jimmunol.1100500 ; PubMed Central PMCID: PMC3119761. - DOI - PMC - PubMed
-
- Derebe MG, Zlatkov CM, Gattu S, Ruhn KA, Vaishnava S, Diehl GE, et al. Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection. Elife. 2014;3:e03206 doi: 10.7554/eLife.03206 ; PubMed Central PMCID: PMC4129439. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

