Histo-Blood Group Antigen Phenotype Determines Susceptibility to Genotype-Specific Rotavirus Infections and Impacts Measures of Rotavirus Vaccine Efficacy
- PMID: 29390150
- PMCID: PMC5894073
- DOI: 10.1093/infdis/jiy054
Histo-Blood Group Antigen Phenotype Determines Susceptibility to Genotype-Specific Rotavirus Infections and Impacts Measures of Rotavirus Vaccine Efficacy
Erratum in
-
Erratum.J Infect Dis. 2018 Jun 5;218(1):171-172. doi: 10.1093/infdis/jiy254. J Infect Dis. 2018. PMID: 29868847 Free PMC article. No abstract available.
Abstract
Background: Lewis and secretor histo-blood group antigens (HBGAs) have been associated with decreased susceptibility to P[8] genotype rotavirus (RV) infections. Efficacy of vaccines containing attenuated P[8] strains is decreased in low-income countries. Host phenotype might impact vaccine efficacy (VE) by altering susceptibility to vaccination or RV diarrhea (RVD). We performed a substudy in a monovalent RV vaccine (RV1) efficacy trial in Bangladesh to determine the impact of Lewis and secretor status on risk of RVD and VE.
Methods: In infants randomized to receive RV1 or no RV1 at 10 and 17 weeks with 1 year of complete active diarrheal surveillance, we performed Lewis and secretor phenotyping and genotyped the infecting strain of each episode of RVD.
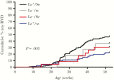
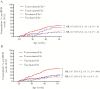
Results: A vaccine containing P[8] RV protected secretors and nonsecretors similarly. However, unvaccinated nonsecretors had a reduced risk of RVD (relative risk, 0.53 [95% confidence interval, .36-.79]) mediated by complete protection from P[4] but not P[8] RVs. This effect reduced VE in nonsecretors to 31.7%, compared to 56.2% among secretors, and decreased VE for the overall cohort.
Conclusions: Host HBGA status may impact VE estimates by altering susceptibility to RV in unvaccinated children; future trials should therefore account for HBGA status.
Clinical trials registration: NCT01375647.
Figures
References
-
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global post-licensure data, 2006–2016. Clin Infect Dis 2017; 65:840–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

