Collecting verbal autopsies: improving and streamlining data collection processes using electronic tablets
- PMID: 29391038
- PMCID: PMC5793369
- DOI: 10.1186/s12963-018-0161-9
Collecting verbal autopsies: improving and streamlining data collection processes using electronic tablets
Abstract
Background: There is increasing interest in using verbal autopsy to produce nationally representative population-level estimates of causes of death. However, the burden of processing a large quantity of surveys collected with paper and pencil has been a barrier to scaling up verbal autopsy surveillance. Direct electronic data capture has been used in other large-scale surveys and can be used in verbal autopsy as well, to reduce time and cost of going from collected data to actionable information.
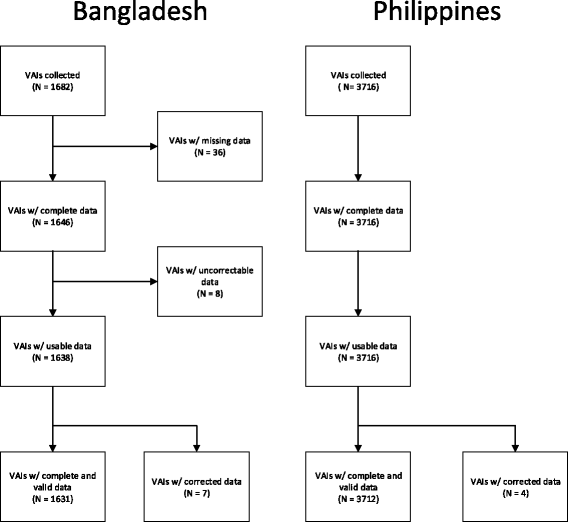
Methods: We collected verbal autopsy interviews using paper and pencil and using electronic tablets at two sites, and measured the cost and time required to process the surveys for analysis. From these cost and time data, we extrapolated costs associated with conducting large-scale surveillance with verbal autopsy.
Results: We found that the median time between data collection and data entry for surveys collected on paper and pencil was approximately 3 months. For surveys collected on electronic tablets, this was less than 2 days. For small-scale surveys, we found that the upfront costs of purchasing electronic tablets was the primary cost and resulted in a higher total cost. For large-scale surveys, the costs associated with data entry exceeded the cost of the tablets, so electronic data capture provides both a quicker and cheaper method of data collection.
Conclusions: As countries increase verbal autopsy surveillance, it is important to consider the best way to design sustainable systems for data collection. Electronic data capture has the potential to greatly reduce the time and costs associated with data collection. For long-term, large-scale surveillance required by national vital statistical systems, electronic data capture reduces costs and allows data to be available sooner.
Conflict of interest statement
Ethics approval and consent to participate
The methods of this study were approved by the Medical Research Ethics Committee of the University of Queensland, Australia; the Institutional Review Board of the Research Institute of Tropical Medicine, Philippines; and by the Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh. All data were collected with informed verbal consent from participants before beginning the interview. This method of consent was approved by the review boards at each site.
Consent for publication
We only reports aggregated statistics. No details, images, or videos relating to individual persons are published with this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

