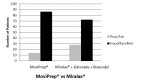
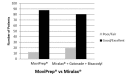
Polyethylene Glycol-3350 (Miralax®)+1.9-L sports drink (Gatorade®)+2 tablets of bisacodyl results in inferior bowel preparation for colonoscopy compared with Polyethylene Glycol-Ascorbic Acid (MoviPrep®)
- PMID: 29391310
- PMCID: PMC6322600
- DOI: 10.5152/tjg.2018.17536
Polyethylene Glycol-3350 (Miralax®)+1.9-L sports drink (Gatorade®)+2 tablets of bisacodyl results in inferior bowel preparation for colonoscopy compared with Polyethylene Glycol-Ascorbic Acid (MoviPrep®)
Abstract
Background/aims: Polyethylene glycol (PEG)-3350, approved by Food and Drug Administration (FDA) only for constipation, combined with 1.9 L of sports drink (SD) (GatoradeR) and bisacodyl (B) is commonly used in outpatient practice for bowel preparation due to cited patient satisfaction and tolerability of this specific regimen. We aim to compare PEG-3350 (MiralaxR) with PEG-AA-based (MoviPrepR) in terms of efficacy, patient satisfaction, and the effects of these two regimen on serum electrolytes.
Materials and methods: This study is a prospective, single-blinded, block randomized trial comparing single-dose PEG-3350+SD+B to split-dose 2-L PEG-AA in the outpatient endoscopy unit in patients undergoing colonoscopy. Basic metabolic profiles were checked on the day of randomization and on the day of procedure. Patients completed a survey on the day of procedure. Bowel preparation quality was assessed using the Boston Bowel Preparation Scale (BBPS) by two endoscopists and a nurse present during the procedure.
Results: We randomized 150 patients (74 PEG-3350+SD+B and 76 PEG-AA). The PEG-AA group had significantly higher BBPS scores in the right colon by Endoscopist 1, Nurse, and Endoscopist 2 (p 0.005, <0.000, 0.001) and in the left and transverse colon by Nurse and Endoscopist 2 (p 0.004, 0.26, 0.000, 0.006). There was no statistically significant difference in patient satisfaction or change in serum electrolytes between the two groups.
Conclusion: Use of single-dose PEG-3350+SD+B results in inferior bowel preparation for colonoscopy compared with split-dose PEGAA and does not provide any advantage in regards to patient satisfaction. We therefore recommend discontinuing the use of PEG 3350 for bowel preparation.
Conflict of interest statement
Figures
Comment in
-
Improving the quality of colonoscopy: Impact of efficient and safer preparation protocols and shorter waiting times.Turk J Gastroenterol. 2018 Jan;29(1):4-6. doi: 10.5152/tjg.2018.18003. Turk J Gastroenterol. 2018. PMID: 29391302 Free PMC article. No abstract available.
Similar articles
-
Comparison of a split-dose bowel preparation with 2 liters of polyethylene glycol plus ascorbic acid and 1 liter of polyethylene glycol plus ascorbic acid and bisacodyl before colonoscopy.Gastrointest Endosc. 2017 Aug;86(2):343-348. doi: 10.1016/j.gie.2016.10.040. Epub 2016 Nov 23. Gastrointest Endosc. 2017. PMID: 27889546 Clinical Trial.
-
Combination of bisacodyl suppository and 1 L polyethylene glycol plus ascorbic acid is a non-inferior and comfortable regimen compared to 2 L polyethylene glycol plus ascorbic acid.Dig Endosc. 2020 May;32(4):600-607. doi: 10.1111/den.13548. Epub 2019 Nov 11. Dig Endosc. 2020. PMID: 31574170 Clinical Trial.
-
2-Litre polyethylene glycol-citrate-simethicone plus bisacodyl versus 4-litre polyethylene glycol as preparation for colonoscopy in chronic constipation.Dig Liver Dis. 2015 Oct;47(10):857-63. doi: 10.1016/j.dld.2015.06.008. Epub 2015 Jul 6. Dig Liver Dis. 2015. PMID: 26232311 Clinical Trial.
-
Miralax with gatorade for bowel preparation: a meta-analysis of randomized controlled trials.Am J Gastroenterol. 2014 Oct;109(10):1566-74. doi: 10.1038/ajg.2014.238. Epub 2014 Aug 19. Am J Gastroenterol. 2014. PMID: 25135007 Review.
-
Navigating Bowel Preparation for Colonoscopy: A Comprehensive Overview.J Clin Gastroenterol. 2025 Apr 1;59(4):285-297. doi: 10.1097/MCG.0000000000002124. J Clin Gastroenterol. 2025. PMID: 39761153 Review.
Cited by
-
Improving the quality of colonoscopy: Impact of efficient and safer preparation protocols and shorter waiting times.Turk J Gastroenterol. 2018 Jan;29(1):4-6. doi: 10.5152/tjg.2018.18003. Turk J Gastroenterol. 2018. PMID: 29391302 Free PMC article. No abstract available.
References
-
- Lewis JD, Gelfand JM, Troxel AB, et al. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1428–35. https://doi.org/10.1111/j.1572-0241.2008.01836.x - DOI - PubMed
-
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. the national polyp study workgroup. N Engl J Med. 1993;329:1977–81. https://doi.org/10.1056/NEJM199312303292701 - DOI - PubMed
-
- Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the american college of gastroenterology. American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–77. https://doi.org/10.1016/S0002-9270(00)00851-0 - DOI - PubMed
-
- Florin TH, Paterson EW, Fowler EV, Radford-Smith GL. Clinically active Crohn’s disease in the presence of a low C-reactive protein. Scand J Gastroenterol. 2006;41:306–11. https://doi.org/10.1080/00365520500217118 - DOI - PubMed
-
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. https://doi.org/10.1056/NEJMoa0907667 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical