Identification of new biomarkers to promote personalised treatment of patients with inflammatory rheumatic disease: protocol for an open cohort study
- PMID: 29391382
- PMCID: PMC5829933
- DOI: 10.1136/bmjopen-2017-019325
Identification of new biomarkers to promote personalised treatment of patients with inflammatory rheumatic disease: protocol for an open cohort study
Abstract
Introduction: The introduction of biological disease-modifying antirheumatic drugs (bDMARDs) has improved the treatment of inflammatory rheumatic diseases dramatically. However, bDMARD treatment failure occurs in 30%-40% of patients due to lack of effect or adverse events, and the tools to predict treatment outcomes in individual patients are currently limited. The objective of the present study is to identify diagnostic, prognostic and predictive biomarkers, which can be used to (1) diagnose inflammatory rheumatic diseases early in the disease course with high sensitivity and specificity, (2) improve prognostication or (3) predict and monitor treatment effectiveness and tolerability for the individual patient.
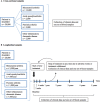
Methods and analysis: The present study is an observational and translational open cohort study with prospective collection of clinical data and biological materials (primarily blood) in patients with inflammatory rheumatic diseases treated in routine care. Patients contribute with one cross-sectional blood sample and/or are enrolled for longitudinal follow-up on initiation of a new DMARD (blood sampling after 0, 3, 6, 12, 24, 36, 48, 60 months of treatment). Other biological materials will be collected when accessible and relevant. Demographics, disease characteristics, comorbidities and lifestyle factors are registered at inclusion; DMARD treatment and outcomes are collected repeatedly during follow-up. Currently (July 2017), >5000 samples from approximately 3000 patients have been collected. Data will be analysed using appropriate statistical analyses.
Ethics and dissemination: The protocol is approved by the Danish Ethics Committee and the Danish Data Protection Agency. Participants give written and oral informed consent. Biomarkers will be evaluated and published according to the Reporting Recommendations for Tumour Marker (REMARK) prognostic studies, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines. Results will be published in peer-reviewed scientific journals and presented at international conferences.
Trial registration number: NCT03214263.
Keywords: biomarkers; danish rheumatologic biobank; inflammatory rheumatic disease; personalised treatment; rheumatology.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
