Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings
- PMID: 29391477
- PMCID: PMC5794763
- DOI: 10.1038/s41598-018-19628-z
Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings
Abstract
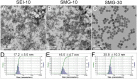
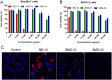
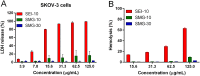
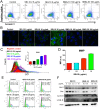
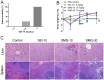
Iron oxide nanoparticles (IONPs) have been increasingly used in biomedical applications, but the comprehensive understanding of their interactions with biological systems is relatively limited. In this study, we systematically investigated the in vitro cell uptake, cytotoxicity, in vivo distribution, clearance and toxicity of commercially available and well-characterized IONPs with different sizes and coatings. Polyethylenimine (PEI)-coated IONPs exhibited significantly higher uptake than PEGylated ones in both macrophages and cancer cells, and caused severe cytotoxicity through multiple mechanisms such as ROS production and apoptosis. 10 nm PEGylated IONPs showed higher cellular uptake than 30 nm ones, and were slightly cytotoxic only at high concentrations. Interestingly, PEGylated IONPs but not PEI-coated IONPs were able to induce autophagy, which may play a protective role against the cytotoxicity of IONPs. Biodistribution studies demonstrated that all the IONPs tended to distribute in the liver and spleen, and the biodegradation and clearance of PEGylated IONPs in these tissues were relatively slow (>2 weeks). Among them, 10 nm PEGylated IONPs achieved the highest tumor uptake. No obvious toxicity was found for PEGylated IONPs in BALB/c mice, whereas PEI-coated IONPs exhibited dose-dependent lethal toxicity. Therefore, it is crucial to consider the size and coating properties of IONPs in their applications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

