Comparison of claims vs patient-reported adherence measures and associated outcomes among patients with nonvalvular atrial fibrillation using oral anticoagulant therapy
- PMID: 29391782
- PMCID: PMC5769561
- DOI: 10.2147/PPA.S148697
Comparison of claims vs patient-reported adherence measures and associated outcomes among patients with nonvalvular atrial fibrillation using oral anticoagulant therapy
Abstract
Objective: To compare oral anticoagulant (OAC) adherence among patients with nonvalvular atrial fibrillation (NVAF) using patient-reported and claims-based measures, and to evaluate the effect of OAC adherence on health care costs and patient satisfaction with OAC therapy.
Methods: This was a hybrid US observational study consisting of a longitudinal cohort survey followed by linkage and analysis of respondents' administrative claims data. Patients with NVAF receiving warfarin, dabigatran, rivaroxaban, or apixaban completed an initial survey and follow-up surveys at 4, 8, and 12 months. Patient-reported adherence was measured at each survey by Morisky Medication Adherence Scale (MMAS-8) and pharmacy claims-determined adherence by the proportion of days covered (PDC) for the 12-month period following the initial survey date; adherence was defined as MMAS-8 score =8 and PDC ≥80%. Patient satisfaction with OAC therapy was assessed by the Duke Anticoagulation Satisfaction Scale (DASS).
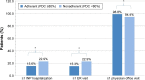
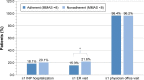
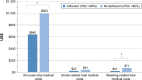
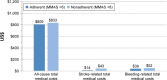
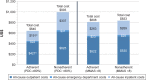
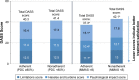
Results: Overall, 675 patients completed at least the initial survey (warfarin, n=271; dabigatran, n=266; rivaroxaban, n=128; apixaban, n=10). Fewer than half (47.9%) were PDC adherent, 37.2% were MMAS-8 adherent, and 19.4% were adherent by both measures. Total medical costs of PDC-adherent patients were significantly lower vs PDC-nonadherent patients (US$640 vs $993 per-patient per-month, respectively, p<0.05). MMAS-8-adherent patients reported higher treatment satisfaction; total DASS score was significantly lower among MMAS-8-adherent than MMAS-8-nonadherent patients (37.3 vs 42.9, respectively, p<0.001).
Conclusion: Using claims-based or patient-reported methods to measure OAC adherence may lead to different results when assessing impact on health care costs and satisfaction with anticoagulation medication. These results underscore the importance of considering both claims-based and patient-reported measures when evaluating treatment adherence in real-world settings.
Keywords: OAC; adherence; atrial fibrillation; health care costs.
Conflict of interest statement
Disclosure WJ Kwong is an employee of Daiichi Sankyo, Inc. JJ Stephenson and H Tan are employees of HealthCore, Inc., a wholly owned subsidiary of Anthem, Inc., which received funding from Daiichi Sankyo, Inc. for the conduct of the study. MU Shinde and A-C Fu were employees of Health-Core, Inc. at the time of the study. WS Weintraub received consulting fees from Daiichi Sankyo, Inc. The authors report no other conflicts of interest in this work.
Figures
References
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. - PubMed
-
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

