Roles of Endoplasmic Reticulum Stress in NECA-Induced Cardioprotection against Ischemia/Reperfusion Injury
- PMID: 29391923
- PMCID: PMC5748120
- DOI: 10.1155/2017/2490501
Roles of Endoplasmic Reticulum Stress in NECA-Induced Cardioprotection against Ischemia/Reperfusion Injury
Abstract
Objective: This study aimed to investigate whether the nonselective A2 adenosine receptor agonist NECA induces cardioprotection against myocardial ischemia/reperfusion (I/R) injury via glycogen synthase kinase 3β (GSK-3β) and the mitochondrial permeability transition pore (mPTP) through inhibition of endoplasmic reticulum stress (ERS).
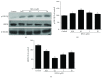
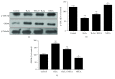
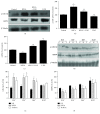
Methods and results: H9c2 cells were exposed to H2O2 for 20 minutes. NECA significantly prevented H2O2-induced TMRE fluorescence reduction, indicating that NECA inhibited the mPTP opening. NECA blocked H2O2-induced GSK-3β phosphorylation and GRP94 expression. NECA increased GSK-3β phosphorylation and decreased GRP94 expression, which were prevented by both ERS inductor 2-DG and PKG inhibitor KT5823, suggesting that NECA may induce cardioprotection through GSK-3β and cGMP/PKG via ERS. In isolated rat hearts, both NECA and the ERS inhibitor TUDCA decreased myocardial infarction, increased GSK-3β phosphorylation, and reversed GRP94 expression at reperfusion, suggesting that NECA protected the heart by inhibiting GSK-3β and ERS. Transmission electron microscopy showed that NECA and TUDCA reduced mitochondrial swelling and endoplasmic reticulum expansion, further supporting that NECA protected the heart by preventing the mPTP opening and ERS.
Conclusion: These data suggest that NECA prevents the mPTP opening through inactivation of GSK-3β via ERS inhibition. The cGMP/PKG signaling pathway is responsible for GSK-3β inactivation by NECA.
Figures





References
-
- Tian Y., Piras B. A., Kron I. L., French B. A., Yang Z. Adenosine 2B receptor activation reduces myocardial reperfusion injury by promoting anti-inflammatory macrophages differentiation via PI3K/Akt pathway. Oxidative Medicine and Cellular Longevity. 2015;2015:8. doi: 10.1155/2015/585297.585297 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

