Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions
- PMID: 29392092
- PMCID: PMC5792238
- DOI: 10.1089/wound.2016.0696
Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions
Abstract
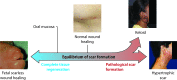
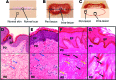
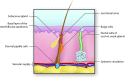
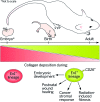
Significance: Scarring of the skin from burns, surgery, and injury constitutes a major burden on the healthcare system. Patients affected by major scars, particularly children, suffer from long-term functional and psychological problems. Recent Advances: Scarring in humans is the end result of the wound healing process, which has evolved to rapidly repair injuries. Wound healing and scar formation are well described on the cellular and molecular levels, but truly effective molecular or cell-based antiscarring treatments still do not exist. Recent discoveries have clarified the role of skin stem cells and fibroblasts in the regeneration of injuries and formation of scar. Critical Issues: It will be important to show that new advances in the stem cell and fibroblast biology of scarring can be translated into therapies that prevent and reduce scarring in humans without major side effects. Future Directions: Novel therapies involving the use of purified human cells as well as agents that target specific cells and modulate the immune response to injury are currently undergoing testing. In the basic science realm, researchers continue to refine our understanding of the role that particular cell types play in the development of scar.
Figures







References
-
- Sheridan RL, et al. Long-term outcome of children surviving massive burns. JAMA 2000;283:69–73 - PubMed
-
- Esselman PC. Burn rehabilitation: an overview. Arch Phys Med Rehabil 2007;88:S3–S6 - PubMed
-
- Robert R, et al. Disfiguring burn scars and adolescent self-esteem. Burns 1999;25:581–585 - PubMed
-
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol 2003;4:245–272 - PubMed
-
- Thomas CR, et al. Personality disorders in young adult survivors of pediatric burn injury. J Pers Disord 2012;26:255–266 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

