The dark side of opioids in pain management: basic science explains clinical observation
- PMID: 29392193
- PMCID: PMC5741356
- DOI: 10.1097/PR9.0000000000000570
The dark side of opioids in pain management: basic science explains clinical observation
Abstract
Introduction: In the past 2 decades, opioids have been used increasingly for the treatment of persistent pain, and doses have tended to creep up. As basic science elucidates mechanisms of pain and analgesia, the cross talk between central pain and opioid actions becomes clearer.
Objectives: We aimed to examine the published literature on basic science explaining pronociceptive opioid actions, and apply this knowledge to clinical observation.
Methods: We reviewed the existing literature on the pronociceptive actions of opioids, both preclinical and clinical studies.
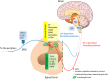
Results: Basic science provides a rationale for the clinical observation that opioids sometimes increase rather than decrease pain. Central sensitization (hyperalgesia) underlies pain chronification, but can also be produced by high dose and high potency opioids. Many of the same mechanisms account for both central pain and opioid hyperalgesia.
Conclusion: Newly revealed basic mechanisms suggest possible avenues for drug development and new drug therapies that could alter pain sensitization through endogenous and exogenous opioid mechanisms. Recent changes in practice such as the introduction of titration-to-effect for opioids have resulted in higher doses used in the clinic setting than ever seen previously. New basic science knowledge hints that these newer dosing practices may need to be reexamined. When pain worsens in a patient taking opioids, can we be assured that this is not because of the opioids, and can we alter this negative effect of opioids through different dosing strategies or new drug intervention?
Keywords: Central sensitization; Hyperalgesia; Opioids; Pain chronification.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Amit Z, Galina ZH. Stress-induced analgesia: adaptive pain suppression. Physiol Rev 1986;66:1091–120. - PubMed
-
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006;104:570–87. - PubMed
-
- Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. PAIN 2003;106:49–57. - PubMed
-
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci 2006;27:558–65. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
