Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 29392288
- PMCID: PMC5885906
- DOI: 10.1001/jamaophthalmol.2017.6565
Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial
Erratum in
-
Error in Abstract and Tables.JAMA Ophthalmol. 2018 May 1;136(5):601. doi: 10.1001/jamaophthalmol.2018.0795. JAMA Ophthalmol. 2018. PMID: 29596563 Free PMC article. No abstract available.
Abstract
Importance: Prevalence of persistent central-involved diabetic macular edema (DME) through 24 weeks of anti-vascular endothelial growth factor therapy and its longer-term outcomes may be relevant to treatment.
Objective: To assess outcomes of DME persisting at least 24 weeks after randomization to treatment with 2.0-mg aflibercept, 1.25-mg bevacizumab, or 0.3-mg ranibizumab.
Design, setting, and participants: Post hoc analyses of a clinical trial, the DRCR.net Protocol T among 546 of 660 participants (82.7%) meeting inclusion criteria for this investigation.
Interventions: Six monthly intravitreous anti-vascular endothelial growth factor injections (unless success after 3 to 5 injections); subsequent injections or focal/grid laser as needed per protocol to achieve stability.
Main outcomes and measures: Persistent DME through 24 weeks, probability of chronic persistent DME through 2 years, and at least 10-letter (≥ 2-line) gain or loss of visual acuity.
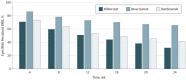
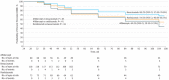
Results: The mean age of participants was 60 years, 363 (66.5%) were white, and 251 (46.0%) were women. Persistent DME through 24 weeks was more frequent with bevacizumab (118 of 180 [65.6%]) than aflibercept (60 of 190 [31.6%]) or ranibizumab (73 of 176 [41.5%]) (aflibercept vs bevacizumab, P < .001; ranibizumab vs bevacizumab, P < .001; and aflibercept vs ranibizumab, P = .05). Among eyes with persistent DME through 24 weeks (n = 251), rates of chronic persistent DME through 2 years were 44.2% with aflibercept, 68.2% with bevacizumab (aflibercept vs bevacizumab, P = .03), and 54.5% with ranibizumab (aflibercept vs ranibizumab, P = .41; bevacizumab vs ranibizumab, P = .16). Among eyes with persistent DME through 24 weeks, proportions with vs without chronic persistent DME through 2 years gaining at least 10 letters from baseline were 62% of 29 eyes vs 63% of 30 eyes (P = .88) with aflibercept, 51% of 70 vs 55% of 31 (P = .96) with bevacizumab, and 45% of 38 vs 66% of 29 (P = .10) with ranibizumab. Only 3 eyes with chronic persistent DME lost at least 10 letters.
Conclusions and relevance: Persistent DME was more likely with bevacizumab than with aflibercept or ranibizumab. Among eyes with persistent DME, eyes assigned to bevacizumab were more likely to have chronic persistent DME than eyes assigned to aflibercept. These results suggest meaningful gains in vision with little risk of vision loss, regardless of anti-vascular endothelial growth factor agent given or persistence of DME through 2 years. Caution is warranted when considering switching therapies for persistent DME following 3 or more injections; improvements could be owing to continued treatment rather than switching therapies.
Trial registration: clinicaltrials.gov Identifier: NCT01627249.
Conflict of interest statement
Figures
Comment in
-
Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema: Does Flavor Matter?JAMA Ophthalmol. 2018 Mar 1;136(3):269-270. doi: 10.1001/jamaophthalmol.2017.6559. JAMA Ophthalmol. 2018. PMID: 29392302 No abstract available.
References
-
- Beck RW, Moke PS, Turpin AH, et al. . A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194-205. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

