A novel patient-derived xenograft model for claudin-low triple-negative breast cancer
- PMID: 29392581
- PMCID: PMC5948145
- DOI: 10.1007/s10549-018-4685-2
A novel patient-derived xenograft model for claudin-low triple-negative breast cancer
Abstract
Background: Triple-negative breast cancer (TNBC) subtypes are clinically aggressive and cannot be treated with targeted therapeutics commonly used in other breast cancer subtypes. The claudin-low (CL) molecular subtype of TNBC has high rates of metastases, chemoresistance and recurrence. There exists an urgent need to identify novel therapeutic targets in TNBC; however, existing models utilized in target discovery research are limited. Patient-derived xenograft (PDX) models have emerged as superior models for target discovery experiments because they recapitulate features of patient tumors that are limited by cell-line derived xenograft methods.
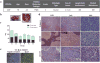
Methods: We utilize immunohistochemistry, qRT-PCR and Western Blot to visualize tumor architecture, cellular composition, genomic and protein expressions of a new CL-TNBC PDX model (TU-BcX-2O0). We utilize tissue decellularization techniques to examine extracellular matrix composition of TU-BcX-2O0.
Results: Our laboratory successfully established a TNBC PDX tumor, TU-BCX-2O0, which represents a CL-TNBC subtype and maintains this phenotype throughout subsequent passaging. We dissected TU-BCx-2O0 to examine aspects of this complex tumor that can be targeted by developing therapeutics, including the whole and intact breast tumor, specific cell populations within the tumor, and the extracellular matrix.
Conclusions: Here, we characterize a claudin-low TNBC patient-derived xenograft model that can be utilized for therapeutic research studies.
Keywords: Claudin-low; Collagen; Decellularized tumor scaffold; Extracellular matrix; Mesenchymal; Patient-derived xenograft; Triple-negative breast cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Alteri R, Bertaut T, Brinton LA, Fedewa S, Freedman RA, Gansler T, et al. Breast cancer facts and figures 2015–2016. Atlanta: American Cancer Society Inc; 2015.
-
- Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLOS Med. 2010 doi: 10.1371/journal.pmed.1000279. - DOI - PMC - PubMed
-
- Crown J, O’Shaughnessy J, Gullo G. Emerging targeted therapies in triple-negative breast cancer. Annals Oncol. 2012;23(S6):vi56–65. - PubMed
-
- Engebraaten O, Vollan HKM, Borresen-Dale A-L. Triple-negative breast cancer and the need for new therapeutic targets. Am J Pathol. 2013;183(4):1064–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

