Preliminary study of microRNA-126 as a novel therapeutic target for primary hypertension
- PMID: 29393351
- PMCID: PMC5810200
- DOI: 10.3892/ijmm.2018.3420
Preliminary study of microRNA-126 as a novel therapeutic target for primary hypertension
Abstract
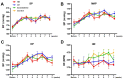
The present study aimed to explore microRNA-126 (miR-126) as a novel therapeutic target for primary hypertension. The lentiviral vector containing human immunodeficiency virus 1 (HIV‑1), the miR‑126 gene knockdown viral vector (lenti-miR-126-KD), and control lentiviral vector (lenti‑scramble‑miR) were constructed. Spontaneously hypertensive rats were randomly divided into 4 groups, which received a high dose of lenti‑miR‑126‑KD (1x108, n=5), low dose of lenti‑miR‑126‑KD (1x107, n=6), scramble‑miR (5x107, n=6), and PBS (n=6). Lentiviral vectors were injected into the tail vein. Data on the systolic blood pressure, diastolic pressure, mean arterial pressure, and heart rate were collected weekly. After 8 weeks of virus administration, the distribution of lentiviral vectors in different tissues was observed by fluorescence microscopy. Picric acid Sirius red and H&E staining were used to observe the target organ damage, and the ELISA kit was used to determine the serum nitric oxide (NO) content. The lentiviral vector was found to be constructed successfully. Eight weeks after the lentiviral vector injection, green fluorescent protein was observed in different tissues in each group. The blood pressure and heart rate were not significantly altered after lentiviral vector injection (P>0.05). No significant differences in the heart‑to‑body weight ratio among the four groups were observed (P=0.23). Picric acid Sirius red and H&E staining revealed that there was no significant difference in morphology among the four groups. No significant difference in the serum NO level among the four groups was noted (P=0.23). The miR‑126 gene knockdown lentiviral vector was constructed successfully. No significant antihypertensive effect was observed by the knockdown of miR‑126 for the treatment of primary hypertension. The target organs were not protected significantly after the treatment. The increased level of miR‑126 expression in hypertensive patients may be due to a compensatory mechanism.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Liu LS, Writing Group of 2010 Chinese Guidelines for the Management of Hypertension 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579–615. In Chinese. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

