Effects of nuclear factor‑κB on the uptake of 131iodine and apoptosis of thyroid carcinoma cells
- PMID: 29393421
- PMCID: PMC5865955
- DOI: 10.3892/mmr.2018.8481
Effects of nuclear factor‑κB on the uptake of 131iodine and apoptosis of thyroid carcinoma cells
Abstract
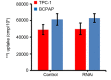
Thyroid carcinoma is primarily treated by surgery combined with radioactive 131iodine (131I) treatment; however, certain patients exhibit resistance to 131I treatment. Previous research indicated that nuclear factor‑κB (NF‑κB) was associated with resistance to 131I in cancer cells. The present study aimed to investigate the effects of NF‑κB on 131I uptake and apoptosis in thyroid carcinoma cells. TPC‑1 and BCPAP cell lines were employed as research models in the present study, and the expression of NF‑κB was inhibited by RNA interference (RNAi). The ability of TPC‑1 and BCPAP cells to uptake 131I was measured and the cell viability was detected by an MTT assay. Finally, the expression of the apoptosis‑associated proteins X‑linked inhibitor of apoptosis (XIAP), cellular inhibitor of apoptosis protein 1 (cIAP1) and caspase‑3 in TCP‑1 and BCPAP cells was determined by western blotting. Western blotting results demonstrated that the expression levels of NF‑κB in TPC‑1 and BCPAP cells were successfully downregulated by RNAi (P<0.05), while analysis of 131I uptake revealed no significant alterations in the 131I uptake ability of cells following RNAi (P>0.05). MTT experiments demonstrated that the inhibition of NF‑κB expression in combination with radiation (131I treatment) led to a marked reduction in cell viability (P<0.05). Furthermore, western blot analysis revealed that the inhibition of NF‑κB expression downregulated the expression levels of XIAP and cIAP1 (P<0.05), while the expression levels of caspase‑3 were upregulated, indicating that the observed reduction in cell viability following NF‑κB inhibition may be due to an increased level of apoptosis. Although NF‑κB inhibition did not affect the 131I uptake of thyroid cancer cells, this inhibition may increase the apoptotic effects of radioactive 131I.
Keywords: thyroid carcinoma; nuclear factor-κB; 131iodine; cell apoptosis; RNA interference.
Figures



References
-
- Londero SC, Krogdahl A, Bastholt L, Overgaard J, Trolle W, Pedersen HB, Bentzen J, Schytte S, Christiansen P, Godballe C, Danish Thyroid Cancer Group Papillary thyroid microcarcinoma in Denmark 1996–2008: A national study of epidemiology and clinical significance. Thyroid. 2013;23:1159–1164. doi: 10.1089/thy.2012.0595. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

