Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges
- PMID: 29394499
- PMCID: PMC5901181
- DOI: 10.1111/bph.14155
Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges
Abstract
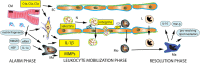
In the infarcted heart, the damage-associated molecular pattern proteins released by necrotic cells trigger both myocardial and systemic inflammatory responses. Induction of chemokines and cytokines and up-regulation of endothelial adhesion molecules mediate leukocyte recruitment in the infarcted myocardium. Inflammatory cells clear the infarct of dead cells and matrix debris and activate repair by myofibroblasts and vascular cells, but may also contribute to adverse fibrotic remodelling of viable segments, accentuate cardiomyocyte apoptosis and exert arrhythmogenic actions. Excessive, prolonged and dysregulated inflammation has been implicated in the pathogenesis of complications and may be involved in the development of heart failure following infarction. Studies in animal models of myocardial infarction (MI) have suggested the effectiveness of pharmacological interventions targeting the inflammatory response. This article provides a brief overview of the cell biology of the post-infarction inflammatory response and discusses the use of pharmacological interventions targeting inflammation following infarction. Therapy with broad anti-inflammatory and immunomodulatory agents may also inhibit important repair pathways, thus exerting detrimental actions in patients with MI. Extensive experimental evidence suggests that targeting specific inflammatory signals, such as the complement cascade, chemokines, cytokines, proteases, selectins and leukocyte integrins, may hold promise. However, clinical translation has proved challenging. Targeting IL-1 may benefit patients with exaggerated post-MI inflammatory responses following infarction, not only by attenuating adverse remodelling but also by stabilizing the atherosclerotic plaque and by inhibiting arrhythmia generation. Identification of the therapeutic window for specific interventions and pathophysiological stratification of MI patients using inflammatory biomarkers and imaging strategies are critical for optimal therapeutic design.
© 2018 The British Pharmacological Society.
Figures
References
-
- Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW et al (2015a). Comparative safety of interleukin‐1 blockade with anakinra in patients with ST‐segment elevation acute myocardial infarction (from the VCU‐ART and VCU‐ART2 pilot studies). Am J Cardiol 115: 288–292. - PubMed
-
- Abbate A, Kontos MC, Grizzard JD, Biondi‐Zoccai GG, Van Tassell BW, Robati R et al (2010). Interleukin‐1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU‐ART] pilot study). Am J Cardiol 105: 1371, e1371–1377. - PubMed
-
- Abbate A, Limana F, Capogrossi MC, Santini D, Biondi‐Zoccai GG, Scarpa S et al (2006). Cyclo‐oxygenase‐2 (COX‐2) inhibition reduces apoptosis in acute myocardial infarction. Apoptosis 11: 1061–1063. - PubMed
-
- Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S et al (2008). Anakinra, a recombinant human interleukin‐1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 117: 2670–2683. - PubMed
-
- Abbate A, Van Tassell BW, Biondi‐Zoccai G, Kontos MC, Grizzard JD, Spillman DW et al (2013). Effects of interleukin‐1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University‐Anakinra Remodeling Trial (2) (VCU‐ART2) pilot study]. Am J Cardiol 111: 1394–1400. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

