CA1 pyramidal neuron gene expression mosaics in the Ts65Dn murine model of Down syndrome and Alzheimer's disease following maternal choline supplementation
- PMID: 29394516
- PMCID: PMC5874173
- DOI: 10.1002/hipo.22832
CA1 pyramidal neuron gene expression mosaics in the Ts65Dn murine model of Down syndrome and Alzheimer's disease following maternal choline supplementation
Abstract
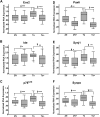
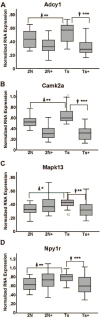
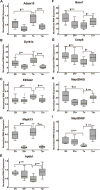
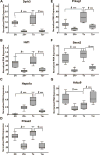
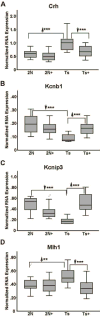
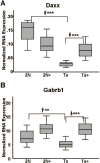
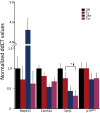
Although there are changes in gene expression and alterations in neuronal density and afferent inputs in the forebrain of trisomic mouse models of Down syndrome (DS) and Alzheimer's disease (AD), there is a lack of systematic assessments of gene expression and encoded proteins within individual vulnerable cell populations, precluding translational investigations at the molecular and cellular level. Further, no effective treatment exists to combat intellectual disability and basal forebrain cholinergic neurodegeneration seen in DS. To further our understanding of gene expression changes before and following cholinergic degeneration in a well-established mouse model of DS/AD, the Ts65Dn mouse, we assessed RNA expression levels from CA1 pyramidal neurons at two adult ages (∼6 months of age and ∼11 months of age) in both Ts65Dn and their normal disomic (2N) littermates. We further examined a therapeutic intervention, maternal choline supplementation (MCS), which has been previously shown to lessen dysfunction in spatial cognition and attention, and have protective effects on the survival of basal forebrain cholinergic neurons in the Ts65Dn mouse model. Results indicate that MCS normalized expression of several genes in key gene ontology categories, including synaptic plasticity, calcium signaling, and AD-associated neurodegeneration related to amyloid-beta peptide (Aβ) clearance. Specifically, normalized expression levels were found for endothelin converting enzyme-2 (Ece2), insulin degrading enzyme (Ide), Dyrk1a, and calcium/calmodulin-dependent protein kinase II (Camk2a), among other relevant genes. Single population expression profiling of vulnerable CA1 pyramidal neurons indicates that MCS is a viable therapeutic for long-term reprogramming of key transcripts involved in neuronal signaling that are dysregulated in the trisomic mouse brain which have translational potential for DS and AD.
Keywords: choline supplementation; hippocampus; laser capture microdissection; microarray; trisomic.
© 2018 Wiley Periodicals, Inc.
Figures
References
-
- ABI. Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR. Applied Biosystems Product Guide. 2004:1–60.
-
- Agrawal R, Tyagi E, Vergnes L, Reue K, Gomez-Pinilla F. Coupling energy homeostasis with a mechanism to support plasticity in brain trauma. Biochim Biophys Acta. 2014;1842:535–546. - PubMed
-
- Akeson EC, Lambert JP, Narayanswami S, Gardiner K, Bechtel LJ, Davisson MT. Ts65Dn – localization of the translocation breakpoint and trisomic gene content in a mouse model for Down syndrome. Cytogenet Cell Genet. 2001;93:270–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

