BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury
- PMID: 29394953
- PMCID: PMC5797353
- DOI: 10.1186/s13287-017-0763-3
BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury
Abstract
Background: Radiation-induced gastrointestinal syndrome (RIGS) results from the acute loss of intestinal stem cells (ISC), impaired epithelial regeneration, and subsequent loss of the mucosal barrier, resulting in electrolyte imbalance, diarrhea, weight loss, sepsis, and mortality. The high radiosensitivity of the intestinal epithelium limits effective radiotherapy against abdominal malignancies and limits the survival of victims of nuclear accidents or terrorism. Currently, there is no approved therapy to mitigate radiation toxicity in the intestine. Here we demonstrate that BCN057, an anti-neoplastic small molecular agent, induces ISC proliferation and promotes intestinal epithelial repair against radiation injury.
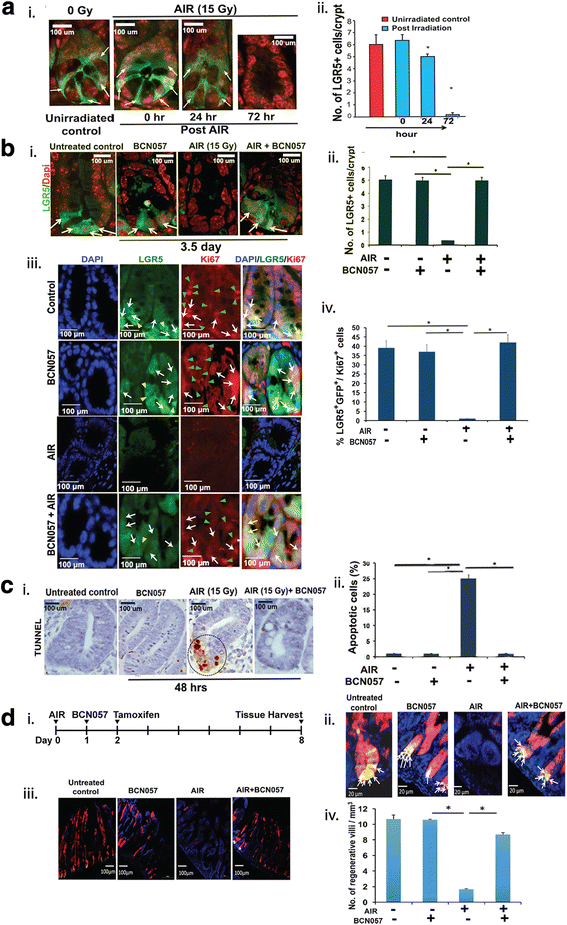
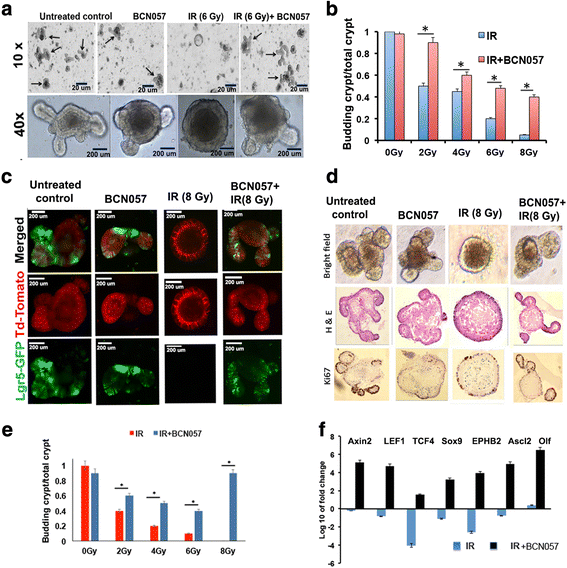
Methods: BCN057 (90 mg/kg body weight, subcutaneously) was injected into C57Bl6 male mice (JAX) at 24 h following abdominal irradiation (AIR) and was continued for 8 days post-irradiation. BCN057-mediated rescue of Lgr5-positive ISC was validated in Lgr5-EGFP-Cre-ERT2 mice exposed to AIR. The regenerative response of Lgr5-positive ISC was examined by lineage tracing assay using Lgr5-EGFP-ires-CreERT2-TdT mice with tamoxifen administration to activate Cre recombinase and thereby marking the ISC and their respective progeny. Ex vivo three-dimensional organoid cultures were developed from surgical specimens of human colon or from mice jejunum and were used to examine the radio-mitigating role of BCN057 on ISC ex vivo. Organoid growth was determined by quantifying the budding crypt/total crypt ratio. Statistical analysis was performed using Log-rank (Mantel-Cox) test and paired two-tail t test.
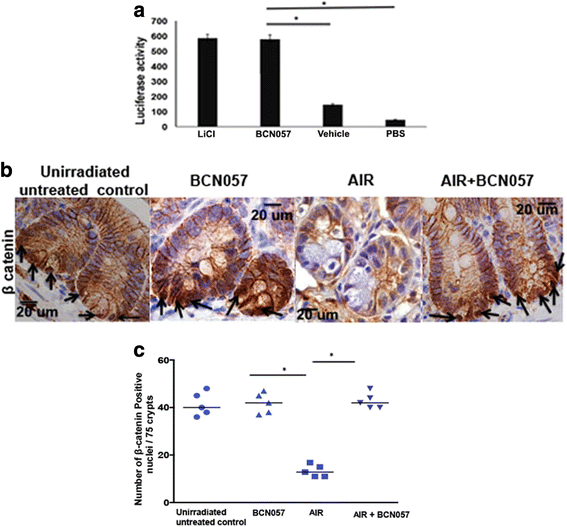
Results: Treatment with BCN057 24 h after a lethal dose of AIR rescues ISC, promotes regeneration of the intestinal epithelium, and thereby mitigates RIGS. Irradiated mice without BCN057 treatment suffered from RIGS, resulting in 100% mortality within 15 days post-radiation. Intestinal organoids developed from mice jejunum or human colon demonstrated a regenerative response with BCN057 treatment and mitigated radiation toxicity. However, BCN057 did not deliver radio-protection to mouse or human colon tumor tissue.
Conclusion: BCN057 is a potential mitigator against RIGS and may be useful for improving the therapeutic ratio of abdominal radiotherapy. This is the first report demonstrating that a small molecular agent mitigates radiation-induced intestinal injury by inducing ISC self-renewal and proliferation.
Keywords: Abdominal radiation; Intestinal stem cell; RIGS; Radiotherapy; Tumor.
Conflict of interest statement
Ethics approval
The present study is not considered as human subject research under HHS regulations at 45 CFR Part 46 and University of Kansas Human Subject Committee as we have used de-identified specimens and no living individual is involved as a study subject.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Saha S, Aranda E, Hayakawa Y, Bhanja P, Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, et al. Macrophage-derived extracellular vesicle-packaged Wnts rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun. 2016;7:13096. doi: 10.1038/ncomms13096. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

