Assessment of the Clinical Relevance of BRCA2 Missense Variants by Functional and Computational Approaches
- PMID: 29394989
- PMCID: PMC5985401
- DOI: 10.1016/j.ajhg.2017.12.013
Assessment of the Clinical Relevance of BRCA2 Missense Variants by Functional and Computational Approaches
Abstract
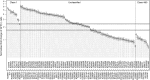
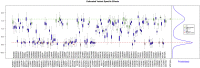
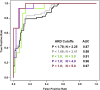
Many variants of uncertain significance (VUS) have been identified in BRCA2 through clinical genetic testing. VUS pose a significant clinical challenge because the contribution of these variants to cancer risk has not been determined. We conducted a comprehensive assessment of VUS in the BRCA2 C-terminal DNA binding domain (DBD) by using a validated functional assay of BRCA2 homologous recombination (HR) DNA-repair activity and defined a classifier of variant pathogenicity. Among 139 variants evaluated, 54 had ?99% probability of pathogenicity, and 73 had ?95% probability of neutrality. Functional assay results were compared with predictions of variant pathogenicity from the Align-GVGD protein-sequence-based prediction algorithm, which has been used for variant classification. Relative to the HR assay, Align-GVGD significantly (p < 0.05) over-predicted pathogenic variants. We subsequently combined functional and Align-GVGD prediction results in a Bayesian hierarchical model (VarCall) to estimate the overall probability of pathogenicity for each VUS. In addition, to predict the effects of all other BRCA2 DBD variants and to prioritize variants for functional studies, we used the endoPhenotype-Optimized Sequence Ensemble (ePOSE) algorithm to train classifiers for BRCA2 variants by using data from the HR functional assay. Together, the results show that systematic functional assays in combination with in silico predictors of pathogenicity provide robust tools for clinical annotation of BRCA2 VUS.
Keywords: BRCA2; VUS; cancer predisposition; functional assay.
Copyright © 2017 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Evers B., Drost R., Schut E., de Bruin M., van der Burg E., Derksen P.W., Holstege H., Liu X., van Drunen E., Beverloo H.B. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin. Cancer Res. 2008;14:3916–3925. - PubMed
-
- Fong P.C., Boss D.S., Yap T.A., Tutt A., Wu P., Mergui-Roelvink M., Mortimer P., Swaisland H., Lau A., O’Connor M.J. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. - PubMed
-
- Audeh M.W., Carmichael J., Penson R.T., Friedlander M., Powell B., Bell-McGuinn K.M., Scott C., Weitzel J.N., Oaknin A., Loman N. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. - PubMed
-
- Tutt A., Robson M., Garber J.E., Domchek S.M., Audeh M.W., Weitzel J.N., Friedlander M., Arun B., Loman N., Schmutzler R.K. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. - PubMed
-
- Gelmon K.A., Tischkowitz M., Mackay H., Swenerton K., Robidoux A., Tonkin K., Hirte H., Huntsman D., Clemons M., Gilks B. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

