Immunogenicity and safety of measles-mumps-rubella vaccine delivered by disposable-syringe jet injector in India: A randomized, parallel group, non-inferiority trial
- PMID: 29395526
- PMCID: PMC5818644
- DOI: 10.1016/j.vaccine.2018.01.006
Immunogenicity and safety of measles-mumps-rubella vaccine delivered by disposable-syringe jet injector in India: A randomized, parallel group, non-inferiority trial
Abstract
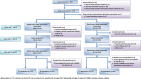
Background: We conducted a randomized, non-inferiority, clinical study of MMR vaccine by a disposable-syringe jet injector (DSJI) in toddlers in India in comparison with the conventional administration.
Methods: MMR vaccine was administered subcutaneously by DSJI or needle-syringe (N-S) to toddlers (15-18 months) who had received a measles vaccine at 9 months. Seropositivity to measles, mumps, and rubella serum IgG antibodies was assessed 35 days after vaccination. Non-inferiority was concluded if the upper limit of the 95% CI for the difference in the percent of seropositive between groups was less than 10%. Solicited reactions were collected for 14 days after vaccination by using structured diaries.
Results: In each study group, 170 subjects received MMR vaccine. On day 35, seropositivity for measles was 97.5% [95% CI (93.8%, 99.3%)] in the DSJI group and 98.7% [95% CI (95.5%, 99.8%)] in the N-S group; for mumps, 98.8% [95% CI (95.6%, 99.8%)] and 98.7% [95% CI (95.5%, 99.8%)]; and for rubella, 98.8% [95% CI (95.6%, 99.8%)] and 100% [95% CI (97.7%, 100.0%)]; none of the differences were significant. The day 35 post-vaccination GMTs in DSJI and N-S groups were measles: 5.48 IU/ml [95% CI (3.71, 8.11)] and 5.94 IU/ml [95% CI (3.92, 9.01)], mumps: 3.83 ISR [95% CI (3.53, 4.14)] and 3.66 ISR [95% CI (3.39, 3.95)] and rubella: 95.27 IU/ml [95% CI (70.39, 128.95)] and 107.06 IU/ml [95% CI (79.02, 145.06)]; none of the differences were significant. The DSJI group reported 173 solicited local reactions and the N-S group reported 112; most were mild grade. Of the total of 156 solicited systemic adverse events, most were mild, and incidence between the two groups was similar.
Conclusions: MMR vaccination via DSJI is as immunogenic as vaccination by N-S. Safety profile of DSJI method is similar to N-S except for injection site reactions which are more with DSJI and are well-tolerated. Registration US National Institutes of Health clinical trials identifier - NCT02253407. Clinical trial registry of India identifier - CTRI/2013/05/003702.
Keywords: Disposable-syringe jet injector (DSJI); Immunogenicity; Measles-mumps-rubella (MMR) vaccine; Needle-free; Safety; Vaccination.
Copyright © 2018 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Immunogenicity and safety of measles-mumps-rubella vaccine delivered by disposable-syringe jet injector in healthy Brazilian infants: a randomized non-inferiority study.Contemp Clin Trials. 2015 Mar;41:1-8. doi: 10.1016/j.cct.2014.11.014. Epub 2014 Dec 1. Contemp Clin Trials. 2015. PMID: 25476584 Clinical Trial.
-
Clinical immunogenicity of measles, mumps and rubella vaccine delivered by the Injex jet injector: comparison with standard syringe injection.Pediatr Infect Dis J. 2000 Sep;19(9):839-42. doi: 10.1097/00006454-200009000-00006. Pediatr Infect Dis J. 2000. PMID: 11001106 Clinical Trial.
-
Immunogenicity and safety of a second dose of a measles-mumps-rubella vaccine administered to healthy participants 7 years of age or older: A phase III, randomized study.Hum Vaccin Immunother. 2018;14(11):2624-2631. doi: 10.1080/21645515.2018.1489186. Epub 2018 Jul 12. Hum Vaccin Immunother. 2018. PMID: 29902133 Free PMC article. Clinical Trial.
-
Measles, mumps, rubella vaccine (Priorix; GSK-MMR): a review of its use in the prevention of measles, mumps and rubella.Drugs. 2003;63(19):2107-26. doi: 10.2165/00003495-200363190-00012. Drugs. 2003. PMID: 12962524 Review.
-
Successes and challenges for preventing measles, mumps and rubella by vaccination.Curr Opin Virol. 2019 Feb;34:110-116. doi: 10.1016/j.coviro.2019.01.002. Epub 2019 Mar 7. Curr Opin Virol. 2019. PMID: 30852425 Review.
Cited by
-
Clinical study of safety and immunogenicity of pentavalent DTP-HB-Hib vaccine administered by disposable-syringe jet injector in India.Contemp Clin Trials Commun. 2019 Jan 9;14:100321. doi: 10.1016/j.conctc.2019.100321. eCollection 2019 Jun. Contemp Clin Trials Commun. 2019. PMID: 30899835 Free PMC article.
-
Evaluation of luciferase and prefusion-stabilized F protein from respiratory syncytial virus mRNA/LNPs in pre-clinical models using jet delivery compared to needle and syringe.Vaccine X. 2023 Dec 9;16:100420. doi: 10.1016/j.jvacx.2023.100420. eCollection 2024 Jan. Vaccine X. 2023. PMID: 38192619 Free PMC article.
-
Measles-Rubella Microarray Patches Phase III Clinical Trial Framework: Proposal and Considerations.Vaccines (Basel). 2024 Nov 6;12(11):1258. doi: 10.3390/vaccines12111258. Vaccines (Basel). 2024. PMID: 39591161 Free PMC article.
-
Vaccination into the Dermal Compartment: Techniques, Challenges, and Prospects.Vaccines (Basel). 2020 Sep 16;8(3):534. doi: 10.3390/vaccines8030534. Vaccines (Basel). 2020. PMID: 32947966 Free PMC article. Review.
-
HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge.Nat Commun. 2019 Feb 18;10(1):798. doi: 10.1038/s41467-019-08739-4. Nat Commun. 2019. PMID: 30778066 Free PMC article.
References
-
- World Health Organization. Measles fact sheet; 2017. http://www.who.int/mediacentre/factsheets/fs286/en/ [accessed 7.12.17].
-
- World Health Organization. Rubella fact sheet; 2017. http://www.who.int/mediacentre/factsheets/fs367/en/ [accessed 7.12.17].
-
- World Health Organization. Recommended routine immunizations for children; 2017. http://www.who.int/immunization/policy/Immunization_routine_table 2.pdf?... [accessed 7.12.17].
-
- World Health Organization (WHO). Global measles and rubella strategic plan 2012–2020. Geneva (Switzerland): WHO; 2012. http://www.who.int/immunization/documents/control/ISBN_978_92_4_150339_6... [accessed 20.10.16].
-
- World Health Organization (WHO). Global vaccine action plan. Monitoring, evaluation & accountability. Secretariat annual report. Geneva (Switzerland): WHO; 2015. http://who.int/immunization/global_vaccine_action_plan/gvap_secretariat_... [accessed 20.10.16].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous