Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management
- PMID: 29395990
- PMCID: PMC5889091
- DOI: 10.1016/S1474-4422(18)30025-5
Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management
Abstract
A coordinated, multidisciplinary approach to care is essential for optimum management of the primary manifestations and secondary complications of Duchenne muscular dystrophy (DMD). Contemporary care has been shaped by the availability of more sensitive diagnostic techniques and the earlier use of therapeutic interventions, which have the potential to improve patients' duration and quality of life. In part 2 of this update of the DMD care considerations, we present the latest recommendations for respiratory, cardiac, bone health and osteoporosis, and orthopaedic and surgical management for boys and men with DMD. Additionally, we provide guidance on cardiac management for female carriers of a disease-causing mutation. The new care considerations acknowledge the effects of long-term glucocorticoid use on the natural history of DMD, and the need for care guidance across the lifespan as patients live longer. The management of DMD looks set to change substantially as new genetic and molecular therapies become available.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DJB was a paid consultant for Hill-Rom Corporation and has US patents (8651107, 8844530, and 9795752) for respiratory devices, as well as related international patents and patent applications. KB was a consultant for Solid Ventures, Catabasis, LGC Ltd, Bristol Myers Squibb, PTC therapeutics, GLC Research, Eli Lilly, and Publicis Life Brands Resolute; she has received grant support from PTC Therapeutics. SDA is a principal investigator for multicentre clinical trials sponsored by PTC Therapeutics and Sarepta Pharmaceuticals. LEC has received personal fees for speaking and participating in research supported by Genzyme Corporation of Sanofi; she has participated in research with CINRG (Cooperative International Neuromuscular Research Group), Enobia Pharma Inc/Alexion, Robertson Foundation, GlaxoSmithKline, Eli Lilly, Valerion, Pfizer, Prosensa, BioMarin, Ionis, Ultragenyx, Roivant Sciences, Therapeutic Research in Neuromuscular Disorders Solutions, NS Pharma, and the Marcus Foundation. DRW is a paid consultant for Health Research Inc and Marathon Pharmaceuticals. LMW has received grant support and honoraria from Novartis and Amgen. All other authors declare no competing interests.
Figures


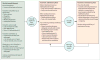



Comment in
-
Evidence-based care in Duchenne muscular dystrophy.Lancet Neurol. 2018 May;17(5):389-391. doi: 10.1016/S1474-4422(18)30115-7. Lancet Neurol. 2018. PMID: 29656735 No abstract available.
References
-
- Bushby K, Finkel R, Birnkrant DJ, et al. for the DMD Care Considerations Working Group Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, et al. for the DMD Care Considerations Working Group Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–89. - PubMed
-
- Gomez-Merino E, Bach JR. Duchenne muscular dystrophy: prolongation of life by noninvasive ventilation and mechanically assisted coughing. Am J Phys Med Rehabil. 2002;81:411–15. - PubMed
-
- Tzeng AC, Bach JR. Prevention of pulmonary morbidity for patients with neuromuscular disease. Chest. 2000;118:1390–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

