B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells
- PMID: 29396161
- PMCID: PMC5821563
- DOI: 10.1016/j.immuni.2018.01.008
B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells
Abstract
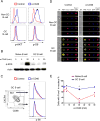
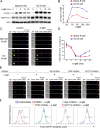
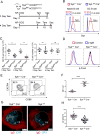
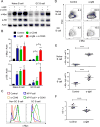
Positive selection of germinal center (GC) B cells is driven by B cell receptor (BCR) affinity and requires help from follicular T helper cells. The transcription factors c-Myc and Foxo1 are critical for GC B cell selection and survival. However, how different affinity-related signaling events control these transcription factors in a manner that links to selection is unknown. Here we showed that GC B cells reprogram CD40 and BCR signaling to transduce via NF-κB and Foxo1, respectively, whereas naive B cells propagate both signals downstream of either receptor. Although either BCR or CD40 ligation induced c-Myc in naive B cells, both signals were required to highly induce c-Myc, a critical mediator of GC B cell survival and cell cycle reentry. Thus, GC B cells rewire their signaling to enhance selection stringency via a requirement for both antigen receptor- and T cell-mediated signals to induce mediators of positive selection.
Keywords: Akt; B cell; B cell receptor; CD40; Foxo1; Myc; Syk; affinity maturation; germinal center.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham- Rundles C, Etzioni A, Franco JL, Gaspar HB, Holland SM, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Frontiers in immunology. 2014;5:162. - PMC - PubMed
-
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007b;315:528–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

