A New Role for the Mitochondrial Pro-apoptotic Protein SMAC/Diablo in Phospholipid Synthesis Associated with Tumorigenesis
- PMID: 29396267
- PMCID: PMC5910671
- DOI: 10.1016/j.ymthe.2017.12.020
A New Role for the Mitochondrial Pro-apoptotic Protein SMAC/Diablo in Phospholipid Synthesis Associated with Tumorigenesis
Abstract
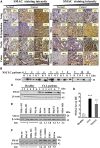
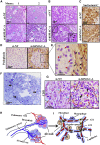
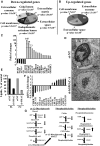
The mitochondrial pro-apoptotic protein SMAC/Diablo participates in apoptosis by negatively regulating IAPs and activating caspases, thus encouraging apoptosis. Unexpectedly, we found that SMAC/Diablo is overexpressed in cancer. This paradox was addressed here by silencing SMAC/Diablo expression using specific siRNA (si-hSMAC). In cancer cell lines and subcutaneous lung cancer xenografts in mice, such silencing reduced cell and tumor growth. Immunohistochemistry and electron microscopy of the si-hSMAC-treated residual tumor demonstrated morphological changes, including cell differentiation and reorganization into glandular/alveoli-like structures and elimination of lamellar bodies, surfactant-producing organs. Next-generation sequencing of non-targeted or si-hSMAC-treated tumors revealed altered expression of genes associated with the cellular membrane and extracellular matrix, of genes found in the ER and Golgi lumen and in exosomal networks, of genes involved in lipid metabolism, and of lipid, metabolite, and ion transporters. SMAC/Diablo silencing decreased the levels of phospholipids, including phosphatidylcholine. These findings suggest that SMAC/Diablo possesses additional non-apoptotic functions related to regulating lipid synthesis essential for cancer growth and development and that this may explain SMAC/Diablo overexpression in cancer. The new lipid synthesis-related function of the pro-apoptotic protein SMAC/Diablo in cancer cells makes SMAC/Diablo a promising therapeutic target.
Keywords: SMAC/Diablo; apoptosis; cancer; mitochondria; phospholipid synthesis.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Diablo: A Double-Edged Sword in Cancer?Mol Ther. 2018 Mar 7;26(3):678-679. doi: 10.1016/j.ymthe.2018.02.004. Epub 2018 Feb 20. Mol Ther. 2018. PMID: 29472100 Free PMC article. No abstract available.
References
-
- Verhagen A.M., Ekert P.G., Pakusch M., Silke J., Connolly L.M., Reid G.E., Moritz R.L., Simpson R.J., Vaux D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. - PubMed
-
- Du C., Fang M., Li Y., Li L., Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. - PubMed
-
- Shiozaki E.N., Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem. Sci. 2004;29:486–494. - PubMed
-
- Verhagen A.M., Vaux D.L. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis. 2002;7:163–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

