A 30-Min Nucleic Acid Amplification Point-of-Care Test for Genital Chlamydia trachomatis Infection in Women: A Prospective, Multi-center Study of Diagnostic Accuracy
- PMID: 29396306
- PMCID: PMC5897918
- DOI: 10.1016/j.ebiom.2017.12.029
A 30-Min Nucleic Acid Amplification Point-of-Care Test for Genital Chlamydia trachomatis Infection in Women: A Prospective, Multi-center Study of Diagnostic Accuracy
Abstract
Background: Rapid Point-Of-Care Tests for Chlamydia trachomatis (CT) may reduce onward transmission and reproductive sexual health (RSH) sequelae by reducing turnaround times between diagnosis and treatment. The io® single module system (Atlas Genetics Ltd.) runs clinical samples through a nucleic acid amplification test (NAAT)-based CT cartridge, delivering results in 30min.
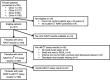
Methods: Prospective diagnostic accuracy study of the io® CT-assay in four UK Genito-Urinary Medicine (GUM)/RSH clinics on additional-to-routine self-collected vulvovaginal swabs. Samples were tested "fresh" within 10days of collection, or "frozen" at -80°C for later testing. Participant characteristics were collected to assess risk factors associated with CT infection.
Results: CT prevalence was 7.2% (51/709) overall. Sensitivity, specificity, positive and negative predictive values of the io® CT assay were, respectively, 96.1% (95% Confidence Interval (CI): 86.5-99.5), 97.7% (95%CI: 96.3-98.7), 76.6% (95%CI: 64.3-86.2) and 99.7% (95%CI: 98.9-100). The only risk factor associated with CT infection was being a sexual contact of an individual with CT.
Conclusions: The io® CT-assay is a 30-min, fully automated, high-performing NAAT currently CE-marked for CT diagnosis in women, making it a highly promising diagnostic to enable specific treatment, initiation of partner notification and appropriately intensive health promotion at the point of care.
Keywords: Chlamydia trachomatis; Diagnostic accuracy; Performance evaluation; Point-of-care; Rapid test; Risk factor.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Abbott Abbott RealTime CT/NG. [Online] 2010. https://www.molecular.abbott/sal/en-us/staticAssets/ctng-8l07-91-us-fina... Package Insert. Available from.
-
- Alonzo T.A., Pepe M.S. Using a combination of reference tests to assess the accuracy of a new diagnostic test. Stat. Med. [Online] 1999;18(22):2987–3003. http://www.ncbi.nlm.nih.gov/pubmed/10544302 Available from. - PubMed
-
- Atkinson L.M., Vijeratnam D., Mani R., Patel R. ‘The waiting game’: are current chlamydia and gonorrhoea near-patient/point-of-care tests acceptable to service users and will they impact on treatment? Int. J. of STD & AIDS. [Online] 2016;27(8):650–655. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

